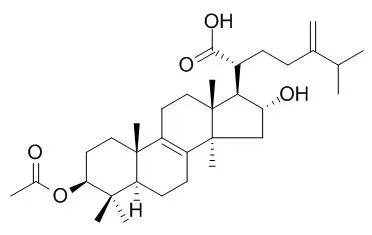
| Description: |
Pachymic acid has sedative-hypnotic, cardioprotective,antioxidant, and anti-inflammatory activities, it has hypoglycemic activity ,can stimulate glucose uptake, GLUT4 gene expression and translocation, and promoting triglyceride accumulation in adipocytes. Pachymic acid may inhibit proliferation and invasion of ovarian carcinoma cell through decreasing β-catenin and COX-2 expression and increasing E-cadherin exprssion. Pachymic acid is suggested to prevent the complications of oral diseases such as inflammation and alveolar destruction of the oral cavity. |
| In vitro: |
| J Endod. 2013 Apr;39(4):461-6. | | The antioxidant property of pachymic acid improves bone disturbance against AH plus-induced inflammation in MC-3T3 E1 cells.[Pubmed: 23522537] | The cytotoxicity of resin-based sealer is influential on the inflammatory reaction and cell survival for oral periapical cells. In this study, Pachymic acid as an antioxidant was investigated for the improvement of bone disturbance against AH Plus (Dentsply DeTrey GmbH, Konstanz, Germany)-induced inflammation in MC-3T3 E1 cells.
METHODS AND RESULTS:
AH Plus was prepared according to the manufacturer's instructions. Using mouse osteoblast cells (MC-3T3 E1), a specimen of AH Plus was eluted with the culture medium for 1 day and was diluted by 30%. The cellular cytotoxicity and reactive oxygen species formation was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and 2',7'-dichlorodihydrofluorescein diacetate with fluorescence-activated cell sorting. The secretion of proinflammatory cytokines was determined by an enzyme-linked immunosorbent assay, and the expression of inflammatory and osteogenic molecules was determined by immunoblotting.
Cells with AH Plus elutes showed a decrease of cell viability and ALP activity. However, Pachymic acid and N-acetyl-L-cysteine (control antioxidant) restored cell viability and ALP activity damaged by AH plus. The secretion of nitric oxide, tumor necrosis factor α, and interleukin-1β were increased in AH Plus-stimulated MC-3T3 E1 cells, but Pachymic acid suppressed its production. Furthermore, Pachymic acid reduced the receptor activator of nuclear factor-κB ligand, cyclooxygenase-2, matrix metalloproteinase-2 and -9, increased bone morphogenetic protein-2 and -7, and runt-related transcription factor 2 despite AH Plus stimuli. In addition, Pachymic acid affected the removal effect of reactive oxygen species formation as did N-acetyl-L-cysteine. More importantly, Pachymic acid inhibited nuclear factor-κB translocation.
CONCLUSIONS:
The property of Pachymic acid can mitigate the unfavorable conditions induced by AH Plus stimuli. Therefore, the use of Pachymic acid is suggested to prevent the complications of oral diseases such as inflammation and alveolar destruction of the oral cavity. | | Eur J Pharmacol. 2010 Dec 1;648(1-3):39-49. | | Pachymic acid stimulates glucose uptake through enhanced GLUT4 expression and translocation.[Pubmed: 20816811 ] | In an effort to investigate the effect and mechanism of Poria cocos on glucose uptake, six lanostane-type triterpenoids were isolated and analyzed.
METHODS AND RESULTS:
Among them, Pachymic acid displayed the most significant stimulating activity on glucose uptake in 3T3-L1 adipocytes. The effect of Pachymic acid on the expression profile of glucose transporters in differentiated 3T3-L1 adipocytes was also analyzed. Our results demonstrated that Pachymic acid induced an increase in GLUT4, but not GLUT1, expression at both the mRNA and protein levels. The role of GLUT4 was further confirmed using the lentiviral vector-derived GLUT4 short hairpin RNA (shRNA). The stimulating activity of Pachymic acid on glucose uptake was abolished when the endogenous GLUT4 expression was suppressed in 3T3-L1 adipocytes. In addition to increased GLUT4 expression, Pachymic acid stimulated GLUT4 redistribution from intracellular vesicles to the plasma membrane in adipocytes. Exposure of the differentiated adipocytes to Pachymic acid increased the phosphorylation of insulin receptor substrate (IRS)-1, AKT and AMP-activated kinase (AMPK). The involvement of PI3K and AMPK in the action of Pachymic acid was further confirmed as PI3K and AMPK inhibitors completely blocked the Pachymic acid-mediated activities in adipocytes. In addition, Pachymic acid was shown to induce triglyceride accumulation and inhibit lipolysis in differentiated adipocytes.
CONCLUSIONS:
Taken together, we demonstrated the insulin-like activities of this compound in stimulating glucose uptake, GLUT4 gene expression and translocation, and promoting triglyceride accumulation in adipocytes. Our study provides important insights into the underlying mechanism of hypoglycemic activity of P. cocos. | | 2015 Oct 15;8(10):17781-8. | | Pachymic acid inhibits tumorigenesis in gallbladder carcinoma cells[Pubmed: 26770369] | | Abstract
Background: Gallbladder cancer, with high aggressivity and extremely poor prognosis, is the most common malignancy of the bile duct. Thus, seeking targets gallbladder tumor cells is an attractive goal towards improving clinical treatment.
Material and methods: In this study, we investigated the effects of Pachymic acid (PA) on the tumorigenesis of human gallbladder cancer cells.
Results: We found that PA significantly reduced cell growth in a dose- and time-dependent fashion. Meanwhile, cell cycle arrest at G0 phase was induced by PA. PA also significantly inhibited cancer cell migration, invasion in a dose-dependent manner. Interestingly, we demonstrated that cancer cell adhesion ability was suppressed dose-dependently, which may contribute to the inhibition of cell invasion. Finally, we showed that PA inhibited AKT and ERK signaling pathways. And oncoproteins, such as PCNA, ICAM-1 and RhoA which are involved intumorigenesis, were also downregulated by PA.
Conclusion: Our study reveals that PA is able to inhibit gallbladder cancer tumorigenesis involving affection of AKT and ERK signaling pathways. Together, these results encourage further studies of PA as a promising candidate for gallbladder cancer therapy.
Keywords: Pachymic acid; adhesion; gallbladder cancer; growth; invasion; migration. |
|
| In vivo: |
| Biomol Ther (Seoul). 2014 Jul; 22(4): 314–20. | | Pachymic Acid Enhances Pentobarbital-Induced Sleeping Behaviors via GABAA-ergic Systems in Mice[Pubmed: 25143810] | This study was investigated to know whether Pachymic acid (PA), one of the predominant triterpenoids in Poria cocos (Hoelen) has the sedative-hypnotic effects, and underlying mechanisms are mediated via γ-aminobutyric acid (GABA)-ergic systems.
METHODS AND RESULTS:
Oral administration of PA markedly suppressed locomotion activity in mice. This compound also prolonged sleeping time, and reduced sleep latency showing synergic effects with muscimol (0.2 mg/kg) in shortening sleep onset and enhancing sleep time induced by pentobarbital, both at the hypnotic (40 mg/kg) and sub-hypnotic (28 mg/kg) doses. Additionally, PA elevated intracellular chloride levels in hypothalamic primary cultured neuronal cells of rats. Moreover, Western blotting quantitative results showed that PA increased the amount of protein level expression of GAD65/67 over a broader range of doses. PA increased α- and β-subunits protein levels, but decreased γ-subunit protein levels in GABAA receptors. The present experiment provides evidence for the hypnotic effects as PA enhanced pentobarbital-induced sleeping behaviors via GABAA-ergic mechanisms in rodents.
CONCLUSIONS:
Taken together, it is proposed that PA may be useful for the treatment of sleep disturbed subjects with insomnia. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)