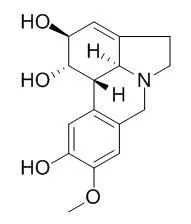
| Description: |
Pseudolycorine, primarily studied as a new antiviral agent , it also shows remarkable antileukemic activity; it can halt HeLa cell growth at 10-1 mM or lower concentrations, it at its growth inhibitory concentrations blocks protein synthesis in ascites cells and stabilize HeLa cell polysomes in vivo. Pseudolycorine and haemanthamine show good activity in in vitro assays against Trypanosoma brucei rhodesiense, T. cruzi and Plasmodium falciparum with IC50 values in the range of 3.66 uM or lower. |
| Targets: |
Antifection | DNA/RNA Synthesis |
| In vitro: |
| J Nat Prod. 2010 Jul 23;73(7):1223-7. | | Amaryllidaceae alkaloids belonging to different structural subgroups display activity against apoptosis-resistant cancer cells.[Pubmed: 20550100] | Fifteen Amaryllidaceae alkaloids (1-15) were evaluated for their antiproliferative activities against six distinct cancer cell lines.
METHODS AND RESULTS:
Several of these natural products were found to have low micromolar antiproliferative potencies. The log P values of these compounds did not influence their observed activity. When active, the compounds displayed cytostatic, not cytotoxic activity, with the exception of Pseudolycorine (3), which exhibited cytotoxic profiles. The active compounds showed similar efficacies toward cancer cells irrespective of whether the cell lines were responsive or resistant to proapoptotic stimuli.
CONCLUSIONS:
Altogether, the data from the present study revealed that lycorine (1), amarbellisine (6), haemanthamine (14), and haemanthidine (15) are potentially useful chemical scaffolds to generate further compounds to combat cancers associated with poor prognoses, especially those naturally resistant to apoptosis, such as glioblastoma, melanoma, non-small-cell lung, and metastatic cancers. | | Phytochem. Lett., 2010, 3(3):161-3. | | In vitro antiprotozoal activity of alkaloids from Phaedranassa dubia (Amaryllidaceae).[Reference: WebLink] | The bulbs of Phaedranassa dubia (Amaryllidaceae) were found to contain the novel compound phaedranamine, together with seven known alkaloids.
METHODS AND RESULTS:
The structure and stereochemistry of the alkaloids were determined by physical and spectroscopic methods. An in vitro screening against four different parasitic protozoa was carried out using the isolated compounds. The alkaloids ungeremine, Pseudolycorine and haemanthamine showed good activity in in vitro assays against Trypanosoma brucei rhodesiense, T. cruzi and Plasmodium falciparum with IC50 values in the range of 3.66 μM or lower. |
|
| In vivo: |
| Proc. Soc. Exp. Biol. Med., 1971 ,136(4): 1168-73. | | Therapeutic Activity of Narcissus Alkaloid on Rauscher Leukemia and Comparison with Standard Drugs[Reference: WebLink] |
METHODS AND RESULTS:
An alkaloid (2-X), tentatively identified as Pseudolycorine, has been isolated from Narcissus tazetta L. This alkaloid, primarily studied as a new antiviral agent derived from the screening of medicinal plants of the Pacific area, has been shown to exert a superior prolongation effect on the life span of established Rauscher leukemic mice having palpable splenomegaly, in comparison with standard antileukemic drugs.
CONCLUSIONS:
It was found that the alkaloid suppressed the development of splenomegaly and the increase in number of nucleated blood cells, and dropped the virus titer in plasma without apparent toxicity.
A second alkaloidal complex, called residual alkaloid, also showed remarkable antileukemic activity. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)