| Kinase Assay: |
| Antimicrob Agents Chemother. 2003 Sep;47(9):2810-6. | | Shikonin, a component of chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1.[Pubmed: 12936978 ] | Shikonin(Shikonine) is a major component of zicao (purple gromwell, the dried root of Lithospermum erythrorhizon), a Chinese herbal medicine with various biological activities, including inhibition of human immunodeficiency virus (HIV) type 1 (HIV-1). G protein-coupled chemokine receptors are used by HIV-1 as coreceptors to enter the host cells.
METHODS AND RESULTS:
In this study, we assessed the effects of shikonin on chemokine receptor function and HIV-1 replication. The results showed that, at nanomolar concentrations, shikonin inhibited monocyte chemotaxis and calcium flux in response to a variety of CC chemokines (CCL2 [monocyte chemoattractant protein 1], CCL3 [macrophage inflammatory protein 1alpha], and CCL5 [regulated upon activation, normal T-cell expressed and secreted protein]), the CXC chemokine (CXCL12 [stromal cell-derived factor 1alpha]), and classic chemoattractants (formylmethionyl-leucine-phenylalanine and complement fraction C5a). Shikonin down-regulated surface expression of CCR5, a primary HIV-1 coreceptor, on macrophages to a greater degree than the other receptors (CCR1, CCR2, CXCR4, and the formyl peptide receptor) did. CCR5 mRNA expression was also down-regulated by the compound. Additionally, shikonin inhibited the replication of a multidrug-resistant strain and pediatric clinical isolates of HIV in human peripheral blood mononuclear cells, with 50% inhibitory concentrations (IC(50)s) ranging from 96 to 366 nM. Shikonin also effectively inhibited the replication of the HIV Ba-L isolate in monocytes/macrophages, with an IC(50) of 470 nM.
CONCLUSIONS:
Our results suggest that the anti-HIV and anti-inflammatory activities of shikonin may be related to its interference with chemokine receptor expression and function. Therefore, shikonin, as a naturally occurring, low-molecular-weight pan-chemokine receptor inhibitor, constitutes a basis for the development of novel anti-HIV therapeutic agents. |
|


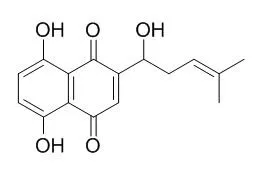



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)