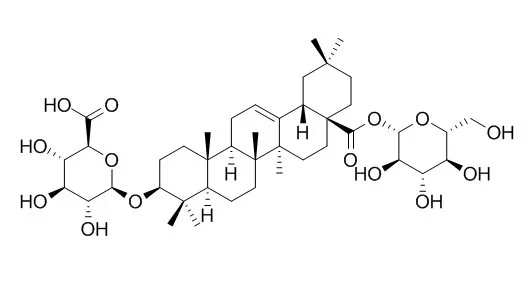
| Description: |
Chikusetsusaponin IVa is a novel AMPK activator, can induce insulin secretion from βTC3 cells via GPR40 mediated calcium and PKC pathways, may be developed into a new potential for therapeutic agent used in T2DM patients.Chikusetsusaponin IVa exerts antithrombotic effects, including minor hemorrhagic events.
|
| Targets: |
AMPK | Wnt/β-catenin | CDK | PKC | GPR | GLUT | Calcium Channel |
| In vitro: |
| Biochem Biophys Res Commun. 2015 Apr 17;459(4):591-6. | | Chikusetsusaponin IVa methyl ester induces cell cycle arrest by the inhibition of nuclear translocation of β-catenin in HCT116 cells.[Pubmed: 25749342] | We demonstrate that Chikusetsusaponin IVa methyl ester (CME), a triterpenoid saponin from the root of Achyranthes japonica, has an anticancer activity.
METHODS AND RESULTS:
We investigate its molecular mechanism in depth in HCT116 cells. CME reduces the amount of β-catenin in nucleus and inhibits the binding of β-catenin to specific DNA sequences (TCF binding elements, TBE) in target gene promoters. Thus, CME appears to decrease the expression of cell cycle regulatory proteins such as Cyclin D1, as a representative target for β-catenin, as well as CDK2 and CDK4. As a result of the decrease of the cell cycle regulatory proteins, CME inhibits cell proliferation by arresting the cell cycle at the G0/G1 phase.
CONCLUSIONS:
Therefore, we suggest that CME as a novel Wnt/β-catenin inhibitor can be a putative agent for the treatment of colorectal cancers. |
|
| In vivo: |
| J Med Food. 2012 Dec;15(12):1073-80. | | Antithrombotic effect of chikusetsusaponin IVa isolated from Ilex paraguariensis (Maté).[Pubmed: 23134458] | The triterpene Chikusetsusaponin IVa was isolated from the fruit of Ilex paraguariensis.
METHODS AND RESULTS:
Using biochemical and pharmacological methods, we demonstrated that Chikusetsusaponin IVa (1) prolongs the recalcification time, prothrombin time, activated partial thromboplastin time, and thrombin time of normal human plasma in a dose-dependent manner, (2) inhibits the amidolytic activity of thrombin and factor Xa upon synthetic substrates S2238 and S2222, (3) inhibits thrombin-induced fibrinogen clotting (50% inhibition concentration, 199.4 ± 9.1 μM), and (4) inhibits thrombin- and collagen-induced platelet aggregation. The results also indicate that Chikusetsusaponin IVa preferentially inhibits thrombin in a competitive manner (K(i)=219.6 μM). Furthermore, when administered intravenously to rats, Chikusetsusaponin IVa inhibited thrombus formation in a stasis model of venous thrombosis, although it did not induce a significant bleeding effect. Chikusetsusaponin IVa also prolonged the ex vivo activated partial thromboplastin time.
CONCLUSIONS:
Altogether, these data suggest that Chikusetsusaponin IVa exerts antithrombotic effects, including minor hemorrhagic events. This appears to be important for the development of new therapeutic agents. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)