| Structure Identification: |
| Zhongguo Zhong Yao Za Zhi. 2013 Nov;38(22):3910-7. | | Chemical constituents from processed rhizomes of Panax notoginseng.[Pubmed: 24558875] |
METHODS AND RESULTS:
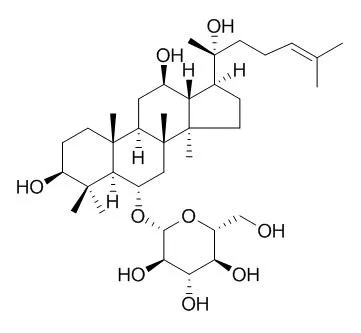
To investigate the chemical constituents of the processed rhizomes of Panax notoginseng, their 70% ethanol extract was chromatographed on macroporous resin (SP825), silica gel, RP-C18 and semi-preparative HPLC to afford compounds 1-23. On the basis of physicochemical properties and spectral data analysis, their structures were identified to be 6'-O-Acetylginsenoside Rh1 (1), ginsenoside RK3 (2), ginsenoside Rh4 (3), 20S-ginsenoside Rg3 (4), ginsenoside Rk1 (5), 20R-ginsenoside Rg3 (6), ginsenoside Rg5 (7), ginsenoside F2 (8), 20S-ginsenoside Rh1 (9), (20R)-Ginsenoside Rh1(10), gypenoside X VII (11), notoginsenoside Fa, (12), ginsenoside Ra3 (13), ginsenoside Rg1 (14), ginsenoside Re (15), notoginsenoside R2 (16), ginsenoside Rg2 (17), notoginsenoside R1 (18), ginsenoside Rd (19), ginsenoside Rb1 (20), notoginsenoside D (21), notoginsenoside R4 (22) and ginsenoside Rb2 (23), respectively. Among them, compound 1 was isolated from P. notoginseng for the first time, and compounds 4, 6, 8 and 11 were isolated from the processed P. notoginseng for the first time.
CONCLUSIONS:
According to the fingerprint profiles of raw and processed P. notoginseng, the putative chemical conversion pathways of panoxatriol and panoxadiol compounds in the processing procedure was deduced, and the results revealed the main reactions to be dehydration and glycosyl hydrolysis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)