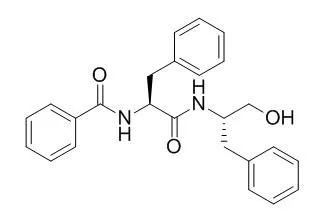
| Description: |
Aurantiamide acetate has anti-cancer, anti-inflammatory and antinociceptive activities, it may suppress the growth of malignant gliomas by blocking autophagic flux, it also inhibits cysteine proteinases, in particular, cathepsin L (3.4.22.15) and B (3.4.22.1) with IC50 of 12 microM and 49 microM, respectively . Aurantiamide acetate has an anti-neuroinflammatory effect on LPS stimulation through its inhibition of the NF-κB, JNK and p38 pathways. |
| In vitro: |
| Int Immunopharmacol. 2014 Dec;23(2):568-74. | | Anti-neuroinflammatory effect of aurantiamide acetate from the marine fungus Aspergillus sp. SF-5921: inhibition of NF-κB and MAPK pathways in lipopolysaccharide-induced mouse BV2 microglial cells.[Pubmed: 25448500] |
METHODS AND RESULTS:
In the course of a search for anti-neuroinflammatory metabolites from marine fungi, Aurantiamide acetate (1) was isolated from marine-derived Aspergillus sp. as an anti-neuroinflammatory component. Compound 1 dose-dependently inhibited the production of nitric oxide (NO) and prostaglandin E2 (PGE2) in BV2 microglial cells. It also attenuated inducible NO synthase (iNOS), cyclooxygenase-2 (COX-2), and other pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). In a further study designed to elucidate the mechanism of its anti-neuroinflammatory effect, Aurantiamide acetate was shown to block the activation of nuclear factor-kappa B (NF-κB) in lipopolysaccharide (LPS)-induced BV2 microglial cells by inhibiting the phosphorylation of the inhibitor kappa B-α (IκB)-α. In addition, Aurantiamide acetate decreased the phosphorylation levels of c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinases (MAPKs).
CONCLUSIONS:
These results suggest that Aurantiamide acetate has an anti-neuroinflammatory effect on LPS stimulation through its inhibition of the NF-κB, JNK and p38 pathways. |
|
| In vivo: |
| J Ethnopharmacol. 2015 Apr 22. | | An in vivo and in vitro assessment of the anti-inflammatory, antinociceptive, and immunomodulatory activities of Clematis terniflora DC. extract, participation of aurantiamide acetate.[Pubmed: 25910534] | Clematis terniflora DC. has been widely used as a traditional Chinese medicine for the treatment of tonsillitis, rheumatoid arthritis, and prostatitis. Despite its widespread use in China, there are currently no studies systematically examined its therapeutic effects and mechanism of action. As such, the present study was conducted to evaluate the anti-inflammatory, antinociceptive, and immunomodulatory effects of C. terniflora DC. using rodent and cellular models.
METHODS AND RESULTS:
The anti-inflammatory properties of the 70% ethanol eluted fraction of the 70% ethanol extract of C. terniflora DC. (EECTD) were evaluated using the xylene-induced ear swelling test, the carrageenan-induced edema model, and the cotton pellet granuloma method. Its antinociceptive activities were determined using both the acetic acid-induced writhing test and the hot plate assay. In parallel, we conducted an in vitro assay in LPS-induced RAW264.7 cells to examine the anti-inflammatory effects of EECTD and its purified form, Aurantiamide acetate (AA) on inhibition of nitric oxide (NO) and prostaglandin E2 (PGE2) release. EECTD (300mg/kg) significantly reduced the number of writhing, extended the pain response latency, and suppressed xylene-induced ear swelling. Each EECTD treatment group also had significant inhibition of cotton granulation formation in addition to reduced carrageenan-induced paw edema. EECTD was also shown to alleviate signs of inflammation in histopathological paw sections. However, it had a less noticeable effect on mouse ear swelling in the delayed type hypersensitivity test. A purified compound was isolated from EECTD and its structure was identified as Aurantiamide acetate. In vitro experimental results showed that both EECTD and Aurantiamide acetate were able to significantly inhibit the release of pro-inflammatory cytokines NO and PGE2 on LPS-induced RAW264.7 cells.
CONCLUSIONS:
These results suggest that EECTD has significant anti-inflammatory and antinociceptive activities, partially related to one of the active substances identified as Aurantiamide acetate. We hypothesize that these effects are related to its ability to inhibit the production of cytokines NO and PGE2. However, further work will be needed to determine its exact mechanism of action. | | Biosci Biotechnol Biochem. 2001 May;65(5):1195-7. | | Aurantiamide acetate, a selective cathepsin inhibitor, produced by Aspergillus penicilloides.[Pubmed: 11440138] |
METHODS AND RESULTS:
Aurantiamide acetate was isolated from the fermentation broth of Aspergillus penicilloides for the first time. Aurantiamide acetate inhibited cysteine proteinases, in particular, cathepsin L (3.4.22.15) and B (3.4.22.1) with IC50 of 12 microM and 49 microM, respectively.
In the adjuvant-arthritic rat model, subcutaneously administered 10 mg/kg body weight of this compound suppressed hind paw swellin. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)