| In vitro: |
| Planta Med 2014; 80 - P2O73 | | The inhibitory effect of chemical constituents derived from Artocarpus xanthocarpus on melanin biosynthesis[Reference: WebLink] |
METHODS AND RESULTS:
Eighteen known compounds and six new compounds were isolated from the roots of Artocarpus xanthocarpus, and their structures were spectroscopically determined. The new compounds included artoxanthocarpuone A (1), artoxanthocarpuone B (2), hydroxylakoochin A (3), methoxylakoochin A (4), epoxylakoochin A (5), and artoxanthol (6). Among the isolated compounds, artoxanthol (6), arboctalol (9) steppogenin (11), norartocarpetin (12), resveratrol (19), oxyresveratrol (20), and Chlorophorin (21) strongly inhibited mushroom tyrosinase activity with IC50 values of 5.7 ± 0.3, 6.4 ± 0.3, 1.9 ± 0.1, 0.9 ± 0.1, 4.9 ± 0.3, 1.0 ± 0.5, and 2.5 ± 0.4μM, respectively.
CONCLUSIONS:
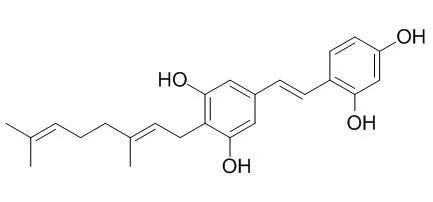
Artoxanthocarpuone A (1), artoxanthocarpuone B (2), methoxylakoochin A (4), lakoochin A (8), cudraflavone C (17), artonin A (18), resveratrol (19), and Chlorophorin (21) reduced tyrosinase activity and inhibited α-melanocyte-stimulating hormone-induced melanin production in B16F10 melanoma cells and were not cytotoxic. Collectively, our results suggest the potential utility of A. xanthocarpus-derived compounds as depigmenting agents. | | J Ethnopharmacol. 2001 Nov;78(1):59-66. | | Anti-amoebic activity of plant compounds from Virgilia oroboides and Chlorophora excelsa.[Pubmed: 11585689] | The anti-amoebic activity of four plant extracts: maackiain and formononetin from Virgilia oroboides and Chlorophorin and Iroko from Chlorophora excelsa, were evaluated.
METHODS AND RESULTS:
Anti-protozoal tests conducted on trophozoites of Entamoeba histolytica established that all four compounds had an affect on the trophozoites to some degree. Chlorophorin showed the highest anti-protozoal activity with an MIC of 0.25 microg/ml followed by maackiain and Iroko with MICs of 1 microg/ml. Chlorophorin and Iroko induced the release of acid phosphatase. Chlorophorin reduced alpha amylase levels by 89%. Formononetin and maackiain had a minimal effect on the enzyme levels. Ultrastructural changes occurred in trophozoites treated with plant compounds. The degree of destruction of the trophozoites increased with an increase in compound concentration. Trophozoite destruction was initiated by the disintegration of the nucleus and culminated with the rupture of the cytoplasmic membrane.
CONCLUSIONS:
Maackiain was the only compound that showed some level of mutagenicity. Formononetin and Iroko were very slightly mutagenic, while Chlorophorin was non-mutagenic. In addition, none of the compounds tested showed cytopathic effects on any of the cell lines tested. Chlorophorin and Iroko exhibit the potential to be exploited as natural multi-functional safe control agents in the treatment of bacterial, fungal and protozoal infections. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)