| In vitro: |
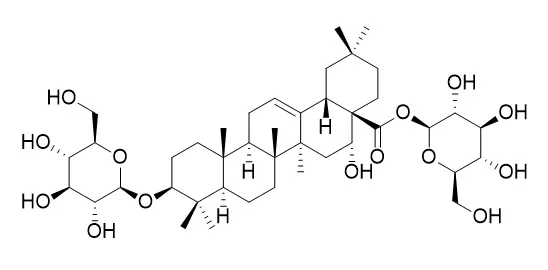
| Food Chem Toxicol. 2012 Nov;50(11):4016-22. | | Eclipta prostrata L. phytochemicals: isolation, structure elucidation, and their antitumor activity.[Pubmed: 22902823] | Eclipta prostrata L., (Asteraceae), is used in China for both food and medicine purposes. This research is concerned with the isolation and purification of phytochemical constituents from the aerial parts of E. prostrata, using gradient solvent fractionation, macroporous resin, silica gel, Sephadex LH-20 and ODS columns, and TLC analyses.
METHODS AND RESULTS:
Four fractions (water, 30% ethanol, 60% ethanol and 90% ethanol) were obtained. Four compounds, wedelolactone (I), Eclalbasaponin I (II), luteolin (III) and luteolin-7-O-glucoside (IV) were purified and their structures were identified by the interpretation of spectroscopic analyses including MS, (1)H and (13)C NMR. Antitumor activities of extracts (total fraction), four fractions and the isolated compounds were assessed using hepatoma cell smmc-7721 as an in vitro assay system. The 30% ethanol fraction and Eclalbasaponin I dose-dependently inhibited the proliferation of hepatoma cell smmc-7721 with IC(50) values of 74.2399 and 111.1703 μg/ml, respectively, more strongly compared with 5-fluorouracil positive control group with the IC(50) value of 195.3131 μg/ml. Antitumor activities of other fractions and compounds were lower than positive control.
CONCLUSIONS:
These results suggested that some specific compounds or extracts from E. prostrata are potential sources of natural anti-tumor materials and worthy of further study. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)