| Structure Identification: |
| J Nat Prod. 2014 Feb 28;77(2):327-38. | | Transformations of the 2,7-Seco Aspidosperma alkaloid leuconolam, structure revision of epi-leuconolam, and partial syntheses of leuconoxine and leuconodines A and F.[Pubmed: 24428198] |
METHODS AND RESULTS:
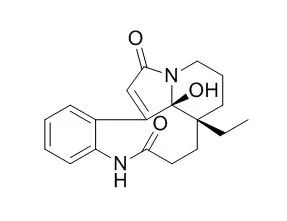
Several transformations of the seco Aspidosperma alkaloid Leuconolam were carried out. The based-induced reaction resulted in cyclization to yield two epimers, the major product corresponding to the optical antipode of a (+)-meloscine derivative. The structures and relative configuration of the products were confirmed by X-ray diffraction analysis. Reaction of Leuconolam and epi-Leuconolam with various acids, molecular bromine, and hydrogen gave results that indicated that the structure of the alkaloid, previously assigned as epi-Leuconolam, was incorrect.
CONCLUSIONS:
This was confirmed by an X-ray diffraction analysis, which revealed that epi-Leuconolam is in fact 6,7-dehydroleuconoxine. Short partial syntheses of the diazaspiro indole alkaloid leuconoxine and the new leuconoxine-type alkaloids leuconodines A and F were carried out. | | Org Lett. 2014 Dec 5;16(23):6216-9. | | Biosynthetically inspired divergent approach to monoterpene indole alkaloids: total synthesis of mersicarpine, leuconodines B and D, leuconoxine, melodinine E, leuconolam, and rhazinilam.[Pubmed: 25412144] |
METHODS AND RESULTS:
Inspired by their potential biosynthesis, we have developed divergent total syntheses of seven monoterpene indole alkaloids including mersicarpine, leuconodines B and D, leuconoxine, melodinine E, Leuconolam, and rhazinilam, and one unnatural analogue with an unprecedented structural skeleton.
CONCLUSIONS:
The key steps involve a Witkop-Winterfeldt oxidative indole cleavage followed by transannular cyclization. The transannular cyclization product was then converted to the corresponding structural skeletons by pairing its functional groups into different reaction modes. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)