| Description: |
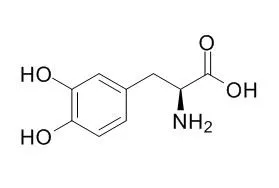
Levodopa is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), used to treat Parkinson's symptoms. |
| Targets: |
Dopamine D3 |
| In vitro: |
| Malays J Med Sci. 2014 Dec;21(Spec Issue):6-11. | | Evaluation of the Cytotoxicity of Levodopa and its Complex with Hydroxypropyl-ß-Cyclodextrin (HP-ß-CD) to an Astrocyte Cell Line.[Pubmed: 25941458] |
METHODS AND RESULTS:
A simple, reliable a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium, (MTS) assay was conducted to evaluate the potential cytotoxic effects of Levodopa, a "gold standard therapy" for Parkinsonism, and its complex with Hydroxypropyl-β-Cyclodextrin (HP-β-CD) on an astrocyte cell line. The cells were incubated in a range of concentrations from 4.69 to 300 μg/mL Levodopa, HP-β-CD or the complex for up to 72 hours. At every 24-hour interval, the optical density (OD), which reflects the number of viable cells, was recorded. In general, linear dose-dependent cytotoxicity profiles were observed for the cells subjected to Levodopa or the complex, whereas a slightly triphasic response was observed for the cells exposed to HP-β-CD. A significant difference (P < 0.05) in cytotoxicity was detected between the HP-β-CD-treated group and the Levodopa-treated group.
CONCLUSIONS:
In particular, we observed that the cells treated with the complex, even at the highest concentrations (> 200 μg/mL), exhibited improved tolerability in a time-dependent manner, which may indicate the potential ability of HP-β-CD to mask the toxic effects of Levodopa via complexation.
|
|
| In vivo: |
| Zhonghua Yi Xue Za Zhi. 2015 Feb 17;95(7):493-5. | | [Effects of a single dose levodopa on heart rate variability in Parkinson's disease].[Pubmed: 25916922] |
To explore the effects of Levodopa on heart rate variability (HRV) in Parkinson's disease (PD).
METHODS AND RESULTS:
A total of 48 PD patients (M:F = 35: 13, mean age: 59 ± 6 years, duration of illness: 4.7 ± 1.8 years, Hoehn & Yahr stage: 2.2 ± 0.3) on a stable dose of Levodopa were recruited from January 2012 to May 2014.For confirming autonomic dysfunction, the baseline patient data (12 hours off-medication) were compared with a control group consisting of 48 age and gender-matched healthy subjects (M: F = 35: 13, mean age 58 ± 6 years). Resting lead II electrocardiogram (ECG) was recorded at baseline and continuously after two tablets of 100/10 mg Levodopa/carbidopa.However, 5-min segments were selected from every quarter, i.e., Q1 (0-15 min), Q2 (15-30 min), Q3 (30-45 min) and Q4 (45-60 min). Artifact-free 5-min segments of ECG were analyzed offline to acquire the parameters of heart rate variability in time and frequency domains.
At baseline, PD patients had a significantly reduced standard deviation of normal-to-normal intervals (SDRR) [(24 ± 4) vs (26 ± 4) ms, P < 0.01)] and total power (TP) [(569 ± 180) vs (652 ± 205) ms2, P < 0.05] when compared to controls. Comparing of HRV in PD patients at baseline and during first hour after drug administration, we observed significant increase in SDRR [(29 ± 12) vs (24 ± 8) ms, P < 0.05)], TP [(708 ± 253) vs (569 ± 180) ms2, P < 0.01], low frequency power (LF) [(203 ± 98) vs (168 ± 60) ms2, P < 0.05) ] and high frequency power (HF) [158 ± 86) vs (114 ± 90) ms2, P < 0.05] in Q3.
CONCLUSIONS:
The results suggest an improvement in the overall variability of heart rate resulting from an enhanced vagal tone. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)