| In vitro: |
| J Food Sci. 2016 May;81(5):M1192-6. | | Antibacterial Constituents of Hainan Morinda citrifolia (Noni) Leaves.[Pubmed: 27074391] | Noni (Morinda citrifolia L.) is an edible and medicinal plant distributed in Hainan, China.
METHODS AND RESULTS:
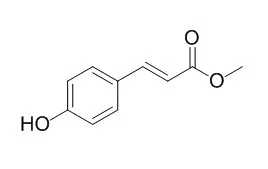
The antibacterial activities of the extracts of water (WE), petroleum ether (PEE), ethyl acetate (EAE), chloroform (CE), and n-butanol (BE) were assayed by the disk diffusion method. The results showed that the extracts from Noni leaves possessed antibacterial effects against Bacillus subtilis, Escherichia coli, Proteus vulgaris, and Staphylococcus aureus. Among 5 different extracts, the BE produced the best antibacterial activity. The samples were first extracted by ethanol, and the primary compounds in the BE fraction of ethanol extract was further isolated and identified. Six phenolic compounds, including 5, 15-dimethylmorindol, ferulic acid, p-hydroxycinamic acid, methyl 4-hydroxybenzoate, methyl ferulate, and Methyl 4-hydroxycinnamate, were identifiedby NMR.
CONCLUSIONS:
The results indicated that the phenolic compounds might significantly contribute to antibacterial activities of Noni leaves. | | J Food Sci. 2017 Aug;82(8):1792-1798. | | Suppression of IL-8 Release by Sweet Olive Ethanolic Extract and Compounds in WiDr Colon Adenocarcinoma Cells.[Pubmed: 28671329] | Oxidative stress can stimulate the secretion of pro-inflammatory cytokines. Interleukin-8 (IL-8) has been implicated in the pathogenesis of inflammatory bowel disease and the metastatic spread of colorectal cancer. The flowers of Osmanthus fragrans (sweet olive) are used to alleviate dysentery with blood in the bowel, as well as stomach ache and diarrhea. However, the evidence of their therapeutic effects on these symptoms remains unclear.
METHODS AND RESULTS:
In the present study, the protective effects of sweet olive flower ethanolic extract (OFE) against oxidative stress in WiDr cells was assessed by evaluating its 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity. In addition, cellular IL-8 secretion was evaluated. Notably, high-performance liquid chromatography showed verbascoside to be the primary constituent in OFE; it exhibited a DPPH scavenging activity with an IC50 of 8.23 μg/mL. Moreover, OFE (1 to 100 μg/mL) showed a potent, dose-dependent inhibitory effect on H2 O2 -induced IL-8 secretion in WiDr cells. Nine compounds were isolated from OFE based on a protective effect-guided purification process. Of these compounds, 5 phenolic compounds-verbascoside, phillygenin, tyrosol, Methyl 4-hydroxycinnamate, and eutigoside A-reduced IL-8 secretion at 10 μg/mL treatment concentrations. Further analysis showed that the anti-inflammatory effects of OFE likely occurred via nuclear factor-κB pathway inhibition, which attenuates IL-8 secretion in cells.
CONCLUSIONS:
Collectively, these data suggest that OFE could be developed as an agent that suppresses IL-8 secretion to treat chronic inflammatory diseases. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)