METHODS AND RESULTS:
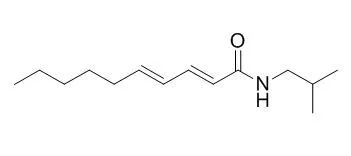
In the present study, ten alkaloids, namely chabamide (1), Pellitorine (2), retrofractamide A (3), pyrroperine (4), isopiperolein B (5), piperamide C9:1 (8E) (6), 6,7-dehydrobrachyamide B (7), 4,5-dihydropiperine (8), dehydropipernonaline (9), and piperine (10), were isolated from the fruits of Piper nigrum. Among these, chabamide (1), Pellitorine (2), retrofractamide A (3), isopiperolein B (5), and 6,7-dehydrobrachyamide B (7) exhibited significant inhibitory activity on lipopolysaccharide-induced nitric oxide (NO) production in RAW264.7 cells, with IC50 values of 6.8, 14.5, 30.2, 23.7, and 38.5 μM, respectively. Furthermore, compound 1 inhibited lipopolysaccharide-induced NO production in bone marrow-derived macrophages with IC50 value of 9.5 μM. Consistent with NO inhibition, treatment of RAW264.7 cells with chabamide (1), Pellitorine (2), and 6,7-dehydrobrachyamide B (7) suppressed expression of inducible NO synthase and cyclooxygenase-2. Chabamide (1), Pellitorine (2), and 6,7-dehydrobrachyamide B (7) induced heme-oxygenase-1 expression at the transcriptional level. In addition, compound 1 induced the nuclear translocation of nuclear factor-E2-related factor 2 (Nrf2) and upregulated the expression of Nrf2 target genes, NAD(P)H:quinone oxidoreductase 1 and γ-glutamyl cysteine synthetase catalytic subunit, in a concentration-dependent manner in RAW264.7 cells.
CONCLUSIONS:
These findings suggest that chabamide (1) from P. nigrum exert antiinflammatory effects via the activation of the Nrf2/heme-oxygenase-1 pathway; hence, it might be a promising candidate for the treatment of inflammatory diseases. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)