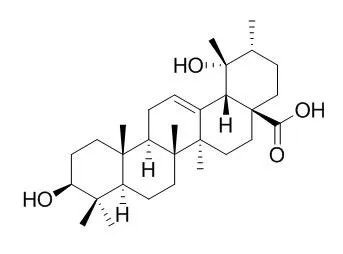
| Description: |
Pomolic acid has anti-cancer, anti-inflammatory and apoptotic activities, it can induce apoptosis in SK-OV-3 cells, which is mediated by the mitochondrial-mediated intrinsic and death receptor-induced extrinsic pathways. Pomolic acid is a potent inhibitor of the aggregation of human platelets induced by ADP and Epinephrine, exhibits IC50 values close to 60 nM and 20 nM, respectively; pomolic acid does not inhibit human platelet aggregation induced by PAF, collagen, U46619 (thromboxane analogue), TRAP or arachidonic acid; suggests that the hypotensive and platelet anti-aggregating effects of pomolic acid and its potential role in cardiovascular therapy. |
| In vitro: |
| J Nat Prod. 1998 Sep;61(9):1090-5. | | Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids.[Pubmed: 9748372] |
METHODS AND RESULTS:
Oleanolic acid (1) was identified as an anti-HIV principle from several plants, including Rosa woodsii (leaves), Prosopis glandulosa (leaves and twigs), Phoradendron juniperinum (whole plant), Syzygium claviflorum (leaves), Hyptis capitata (whole plant), and Ternstromia gymnanthera (aerial part). It inhibited HIV-1 replication in acutely infected H9 cells with an EC50 value of 1.7 microg/mL, and inhibited H9 cell growth with an IC50 value of 21.8 microg/mL [therapeutic index (T. I.) 12.8]. Pomolic acid, isolated from R. woodsii and H. capitata, was also identified as an anti-HIV agent (EC50 1.4 microg/mL, T. I. 16.6). Although ursolic acid did show anti-HIV activity (EC50 2.0 microg/mL), it was slightly toxic (IC50 6.5 microg/mL, T. I. 3.3). A new triterpene (11) was also isolated from the CHCl3-soluble fraction of R. woodsii, though it showed no anti-HIV activity. The structure of 11 was determined to be 1beta-hydroxy-2-oxoPomolic acid by spectral examination. Based on these results, we examined the anti-HIV activity of oleanolic acid- or Pomolic acid-related triterpenes isolated from several plants. In addition, we previously demonstrated that derivatives of betulinic acid, isolated from the leaves of S. claviflorum as an anti-HIV principle, exhibited extremely potent anti-HIV activity.
CONCLUSIONS:
Accordingly, we prepared derivatives of oleanolic acid and evaluated their anti-HIV activity. Among the oleanolic acid derivatives, 18 demonstrated most potent anti-HIV activity, with an EC50 value of 0. 0005 microg/mL and a T. I. value of 22 400. | | Phytomedicine. 2012 Apr 15;19(6):484-7. | | Pomolic acid, triterpenoid isolated from Licania pittieri, as competitive antagonist of ADP-induced aggregation of human platelets.[Pubmed: 22402243] | Pomolic acid (PA), triterpenoid isolated from Licania pittieri, has previously shown a potent ability to inhibit adenosine diphosphate (ADP)- and epinephrine-induced human platelet aggregation.
METHODS AND RESULTS:
To investigate whether Pomolic acid could be an antagonist of ADP-activated receptors of human platelets (P2Y(1) and P2Y(12)), pharmacological studies were conducted to examining its ability to modulate the platelet shape change induced by a selective P2Y(1) receptor agonist MRS2365 and also the nature of its possible interaction with ADP receptors by analyzing the characteristics of log concentration-response curves of ADP constructed in the absence and in the presence of fixed concentrations of Pomolic acid , using in vitro platelet aggregation assays. Pomolic acid did not interfere with the activation of P2Y(1) receptor by MRS2365 to induce platelet shape change and displayed a competitive antagonism of ADP-induced platelet aggregation, which most probably involves competition for a single binding site in platelets. The estimated equilibrium dissociate constant (K(b)) of Pomolic acid as ADP receptor antagonist was 15.4±0.06nM.
CONCLUSIONS:
Together, these findings give indirect evidence for the idea that Pomolic acid could be a potent competitive antagonist of P2Y(12) receptor, and open the possibility to consider it as new member of the non-nucleotide generation of antiplatelet drugs. | | Oncol Rep . 2017 Oct;38(4):2525-2534. | | Pomolic acid induces apoptosis and inhibits multidrug resistance protein MRP1 and migration in glioblastoma cells[Pubmed: 28849227] | | Abstract
Glioblastoma (GBM), the most aggressive of primary brain tumors, determine short survival and poor quality of life. Therapies used for its treatment are not effective and chemotherapy failure is partially due to multidrug resistance (MDR) mechanisms present in the tumor cells. New therapeutic strategies are needed in order to improve survival in GBM. The present study investigated the activity of the pentacyclic triterpene Pomolic acid (PA) in GBM. Pomolic acid decreased the viability and induced apoptosis of GBM cells as demonstrated by DNA fragmentation. It also induced uncoupling of mitochondria membrane potential and activation of caspase-3 and -9. Pomolic acid-induced apoptosis is dependent on reactive oxygen species (ROS) production as it is inhibited by anti-oxidant treatment. Pomolic acid also down-modulated the activity of the multidrug resistance associated protein 1 (MRP1) and inhibited migration of GBM cells. These results show that PA acts on several pathways of GBM drug resistance and therefore may be of potential interest for the treatment of this tumor. | | Molecules . 2018 Sep 3;23(9):2236. | | Pomolic Acid Ameliorates Fibroblast Activation and Renal Interstitial Fibrosis through Inhibition of SMAD-STAT Signaling Pathways[Pubmed: 30177595] | | Abstract
Fibrosis is a common pathological feature in most kinds of chronic kidney disease. Transforming growth factor β1 (TGF-β1) signaling is the master pathway regulating kidney fibrosis pathogenesis, in which mothers against decapentaplegic homolog 3 (SMAD3) with signal transducer and activator of transcription 3 (STAT3) act as the integrator of various pro-fibrosis signals. We examine the effects of Pomolic acid (PA) on mice with unilateral ureteral obstruction (UUO) and TGF-β1 stimulated kidney fibroblast cells. UUO mice were observed severe tubular atrophy, and tubulointerstitial fibrosis and extracellular matrix (ECM) deposition at seven days postoperatively. However, PA-treated UUO mice demonstrated only moderate injury, minimal fibrosis, and larger reductions in the expression of ECM protein and epithelial-mesenchymal transition (EMT) progress. PA inhibited the SMAD-STAT phosphorylation in UUO mice. PA effects were also confirmed in TGF-β1 stimulated kidney fibroblast cells. In this study, we first demonstrated that PA ameliorates fibroblast activation and renal interstitial fibrosis. Our results indicate that PA may be useful as a potential candidate in the prevention of chronic kidney disease.
Keywords: ECM; Pomolic acid; Renal fibrosis; TGF-β1; fibroblast. |
|
| In vivo: |
| Phytomedicine. 2011 Apr 15;18(6):464-9. | | Pomolic acid of Licania pittieri elicits endothelium-dependent relaxation in rat aortic rings.[Pubmed: 21112754] | Pomolic acid has recently shown hypotensive effect in rats. The purpose of this investigation was to determine the vascular effects of this triterpenoid and to examine its mode of action.
METHODS AND RESULTS:
Functional experiments in rat aortic rings precontracted with norepinephrine were performed to evaluate the vasorelaxant effect of Pomolic acid. This triterpenoid induced a vasorelaxation (IC₅₀ = 2.45 μM) in a concentration- and endothelium-dependent manner and showed no effect on contractions evoked by KCl (25 mM). Pre-treatment of aortic rings with L-NAME (100 μM), methylene blue (100 μM) or glibenclamide (10 μM), totally prevented the vasorelaxation induced by Pomolic acid, while indomethacin (10 μM) had no effect on this response. Additionally, Pomolic acid relaxation was unaffected under the muscarinic- and β-adrenergic-receptor blocked ensured for atropine and propanolol respectively (10 μM each). In contrast, the vasorelaxant effect of Pomolic acid was abolished under the purinergic-receptor blocked ensured for suramin (10 μM). Finally, apyrase (0.8 U/ml) an enzyme which hydrolyses ATP and ADP did not affect Pomolic acid relaxation.
CONCLUSIONS:
In summary, Pomolic acid has a potent endothelium-dependent vasorelaxant effect, possibly acting through the direct activation of endothelial purinergic receptors via NO-cGMP signaling pathway, which could be part of the mechanism underlying its hypotensive effect. | | Planta Med. 2008 Feb;74(3):215-20. | | Anti-inflammatory and apoptotic activities of pomolic acid isolated from Cecropia pachystachya.[Pubmed: 18260049] |
METHODS AND RESULTS:
The dichloromethane extract and Pomolic acid ( 5) obtained from leaves of Cecropia pachystachya both reduced carrageenan-induced paw oedema in mice. Interestingly, while the triterpenoid inhibited the in vivo production of interleukin-1beta by 39 %, it had no effect on tumour necrosis factor-alpha production. We also demonstrated that both the dichloromethane extract and 5 inhibited the viability of human polymorphonuclear (PMN) cells in a time- and dose-dependent fashion. The PMN membrane integrity was determined with the aid of flow cytometry by means of the exclusion of propidium iodide as assay. Although the cell membrane integrity was altered, neither the extract nor 5 produced cellular necrosis. Moreover, the development of hypodiploid nuclei and DNA fragmentation in the PMN cells were both dependent on dose and time. Finally, in the annexin V-FITC binding assay, compound 5 increased the total of apoptotic cells by 42 % at 100 microM and by 71 % at 200 microM with respect to the control group.
CONCLUSIONS:
In conclusion, both the dichloromethane extract of ambay and isolated compound 5 inhibit the viability of PMN cells through apoptosis. Since they can regulate human neutrophil functions in this way, it is likely that these substances can also limit inflammation. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)