| In vitro: |
| J Pharm Biomed Anal. 2014 Jul;95:113-20. | | In vitro metabolism of the alkaloid piplartine by rat liver microsomes.[Pubmed: 24667565] | Because Piplartine (PPT) has demonstrated biological activities, such as cytotoxic, anxiolytic, antidepressant, antifungal and antiplatelet activities, this molecule is a relevant drug candidate. The metabolic fate of drug candidates is an essential requirement in assessing their safety and efficacy. Based on this requirement, the biotransformation of PPT by cytochrome P450 enzymes (CYP) was investigated for the first time.
METHODS AND RESULTS:
To determine the in vitro enzymatic kinetic parameters, an HPLC method was developed and validated to quantify PPT. All samples were separated on a reversed-phase C18 column using a mobile phase of acetonitrile:water (40:60, v/v). The method exhibited a linear range of 2.4-157.7 μmol/L, with the following calibration curve: y=0.0934 (±0.0010)x+0.0027, r=0.9975. The lower limit of quantitation was verified to be 2.4 μmol/L, with an RSD below 7%. The precision and accuracy were assessed for both within-day and between-day determinations; neither relative standard (RSD%) deviations nor relative errors (RER) exceeded a value of 15%. The mean absolute recovery was 85%, with an RSD value below 6%. The enzymatic kinetic parameters revealed a sigmoidal profile, with V(max)=4.7±0.3 μmol/mg mL⁻¹/min, h=2.5±0.4, S₅₀=44.7±0.3 μmol/L and CL(max)=0.054 μL/min/mg protein, indicating cooperativity behavior. Employing a mammalian model, PPT metabolism yielded two unreported monohydroxylated products (m/z 334). The identification and structural elucidation of the metabolites were performed by comparing their mass spectra with those spectra of the parent drug.
CONCLUSIONS:
For the first time, the in vitro metabolism studies employing microsomes were demonstrated to be a suitable tool for data regarding enzymatic kinetics and for the metabolites formed in the PPT mammalian metabolism. | | Planta Med. 2015 Jan;81(1):15-9. | | Antitumour efficacy of Piper tuberculatum and piplartine based on the hollow fiber assay.[Pubmed: 25519832 ] | Piper tuberculatum, popularly known in Brazil as "jaborandi falso" and "pimenta darta", is widely used in folk medicine for the treatment of several diseases.
METHODS AND RESULTS:
In this study, the in vivo hollow fiber assay was used to investigate the antitumour efficacy of the crude extract and Piplartine obtained from P. tuberculatum roots. Human glioblastoma (SF-295) and colon carcinoma (HCT-8) cell lines were used. In vitro cytotoxicity was assayed by the MTT assay. In the hollow fiber assay, nude mice implanted with tumour cells in hollow fibers were treated for four consecutive days via the intraperitoneal route, and tumour cell populations were assessed by the MTT assay.
Both the crude extract and Piplartine displayed cytotoxicity. In the hollow fiber assay, tumour growth inhibition rates were 24.6-54.8 % for the crude extract and 33.7-62.2 % for Piplartine. No signal of toxicity was noticed.
CONCLUSIONS:
In conclusion, the crude extract and Piplartine obtained from P. tuberculatum roots displayed in vitro and in vivo anticancer efficacy. | | Chem Biol Drug Des. 2016 Jun;87(6):833-40. | | Design, Synthesis and Pharmacological Evaluation of Novel Piperlongumine derivatives as Potential Antiplatelet Aggregation Candidate.[Pubmed: 26706668 ] |
METHODS AND RESULTS:
A series of novel piperlongumine derivatives (4a-i, 6a-i) were designed and synthesized. The inhibitory activities of platelet aggregation induced by ADP and AA in vitro have been evaluated by bron turbidimetry and liver microsomal incubated assay.
CONCLUSIONS:
The assay results show that compounds 4e and 6e exhibited remarkable potency to that of the positive control Piplartine and aspirin. |
|
| In vivo: |
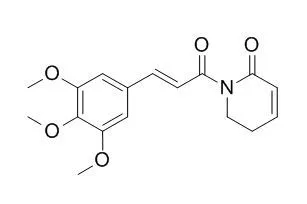
| Mutat Res. 2009 Jun-Jul;677(1-2):8-13. | | Piplartine induces genotoxicity in eukaryotic but not in prokaryotic model systems.[Pubmed: 19379832 ] | Piplartine {5,6-dihydro-1-[(2E)-1-oxo-3-(3,4,5-trimethoxyphenyl)-2-propen-1-yl]-2(1H)-pyridinone} is an alkamide present in Piper species that exhibits promising anticancer properties. It was previously shown that Piplartine is mutagenic in yeast and cultured mammalian cells.
METHODS AND RESULTS:
This study was performed to increase the knowledge on the mutagenic potential of Piplartine using the Salmonella/microsome assay, V79 cell micronucleus and chromosome aberration assays, and mouse bone-marrow micronucleus tests. Piplartine was isolated from the roots of Piper tuberculatum. This extracted compound was unable to induce a mutagenic response in any Salmonella typhimurium strain either in the presence or absence of metabolic activation. Piplartine showed mutagenic effects in V79 cells, as there was an increased frequency of aberrant cells and micronuclei formation. In addition, Piplartine administered at 50mg/kg did not induce micronucleus formation in vivo, but a dose of 100mg/kg induced an increase in the levels of micronucleus polychromatic erythrocytes (MNPCEs).
CONCLUSIONS:
Overall, these results provide further support that Piplartine induces in vivo and in vitro mutagenicity in eukaryotic models. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)