| Kinase Assay: |
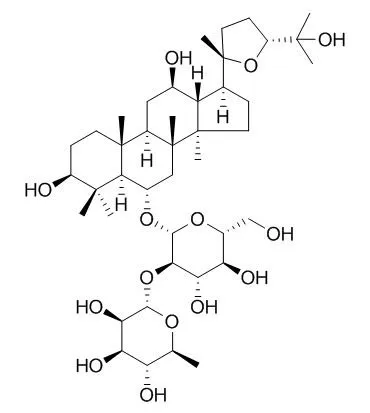
| PPAR Res. 2013;2013:701017. | | Pseudoginsenoside F11, a Novel Partial PPAR γ Agonist, Promotes Adiponectin Oligomerization and Secretion in 3T3-L1 Adipocytes.[Pubmed: 24454336] | PPAR γ is a nuclear hormone receptor that functions as a master regulator of adipocyte differentiation and development. Full PPAR γ agonists, such as the thiazolidinediones (TZDs), have been widely used to treat type 2 diabetes. However, they are characterized by undesirable side effects due to their strong agonist activities. Pseudoginsenoside F11 (p-F11) is an ocotillol-type ginsenoside isolated from Panax quinquefolium L. (American ginseng).
METHODS AND RESULTS:
In this study, we found that p-F11 activates PPAR γ with modest adipogenic activity. In addition, p-F11 promotes adiponectin oligomerization and secretion in 3T3-L1 adipocytes. We also found that p-F11 inhibits obesity-linked phosphorylation of PPAR γ at Ser-273 by Cdk5.
CONCLUSIONS:
Therefore, p-F11 is a novel partial PPAR γ agonist, which might have the potential to be developed as a new PPAR γ -targeted therapeutics for type 2 diabetes. |
|
| Animal Research: |
| Pharmacol Biochem Behav. 2013 May;106:57-67. | | Anti-amnesic effect of pseudoginsenoside-F11 in two mouse models of Alzheimer's disease.[Pubmed: 23541491] | Alzheimer's disease (AD) is a progressive neurodegenerative disease characterized by amyloid β (Aβ) deposits, elevated oxidative stress, and apoptosis of the neurons. Pseudoginsenoside F11 (PF11), a component of Panax quinquefolium (American ginseng), has been demonstrated to antagonize the learning and memory deficits induced by scopolamine, morphine and methamphetamine in mice.
METHODS AND RESULTS:
In the present study, we investigated the effect of Pseudoginsenoside F11 on AD-like cognitive impairment both in mice induced by intracerebroventricular injection of Aβ1-42 (410 pmol) and in Tg-APPswe/PS1dE9 (APP/PS1) mice. It was found that oral treatment with Pseudoginsenoside F11 significantly mitigated learning and memory impairment in mice given Aβ1-42-treated mice for 15 days at doses of 1.6 and 8 mg/kg and APP/PS1 for 4 weeks at a dose of 8 mg/kg as measured by the Morris water maze and step-through tests. In APP/PS1 mice, Pseudoginsenoside F11 8 mg/kg significantly inhibited the expressions of β-amyloid precursor protein (APP) and Aβ1-40 in the cortex and hippocampus, restored the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) and decreased the production of malondialdehyde (MDA) in the cortex. It also noticeably improved the histopathological changes in the cortex and hippocampus and downregulated the expressions of JNK 2, p53 and cleaved caspase 3 in the hippocampus.
CONCLUSIONS:
These findings suggested that the inhibitory effect on amyloidogenesis and oxidative stress and some beneficial effects on neuronal functions might contribute to the recognition improvement effect of Pseudoginsenoside F11 in APP/PS1 mice. Cumulatively, the present study indicated that Pseudoginsenoside F11 may serve as a potential therapeutic agent for the treatment of AD. | | Pharmacol Biochem Behav. 2000 Jul;66(3):595-601. | | Antagonistic effect of pseudoginsenoside F11 on the behavioral actions of morphine in mice.[Pubmed: 10899376] |
METHODS AND RESULTS:
The antagonistic effect of pseudoginoside-F11 (PF(11)) on the various actions of morphine was studied in mice. The results demonstrated that PF(11), at the doses of 4 and 8 mg/kg, PO, significantly inhibited morphine (10 mg/kg, SC)-induced memory impairment in the Morris water maze test. PF(11), at 4 mg/kg, PO, did not influence conditioned place preference per se, yet markedly blocked the conditioned place preference to morphine. PF(11), at the doses of 4 and 8 mg/kg, PO, also significantly antagonized morphine (5 mg/kg, SC)-induced analgesia tested by tail pinch method. PF(11), at 4 mg/kg, PO, did not influence locomotor activity per se, yet inhibited the development of the reverse tolerance, as shown by the increase in locomotor activity, to morphine. At the doses of 4 and 8 mg/kg, PO, PF(11) significantly antagonized the development of analgesia tolerance to morphine in the tail pinch test.
CONCLUSIONS:
Thus, the above results demonstrate for the first time that PF(11) can antagonize some actions of morphine. However, the mechanism of action of PF(11) merits further evaluation. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)