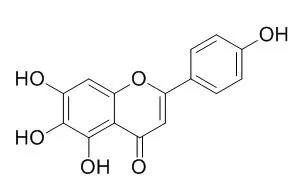
| Description: |
Scutellarein has antioxidant, antitumor, anti-adipogenic, antiviral and anti-inflammatory activities, it can improve neuronal injury, has better protective effect in rat cerebral ischemia. Scutellarein may serve as a SARS-CoV chemical inhibitor, it also exerts strong inhibition towards the tested UDP-glucuronosyltransferase isoforms. |
| In vitro: |
| Chin J Physiol. 2014 Aug 31;57(4):182-7. | | Inhibitory effects of scutellarein on proliferation of human lung cancer A549 cells through ERK and NFκB mediated by the EGFR pathway.[Pubmed: 25246059] | High expression levels of cyclooxygenase-2 (COX-2) contribute a strong proliferative ability to human lung cancer cells, and this function is link to the epidermal growth factor receptor (EGFR) pathway, which was mediated by extracellular-signal-regulated kinase (ERK) and nuclear factor kappa B (NFκB).
METHODS AND RESULTS:
In this study, Scutellarein, a flavonoid compound, was screened for proliferation inhibition at different concentrations (0, 5, 25 and 50 μM) at 24 h or 48 h in human lung cancer cell line A549. Results showed that A549 cell proliferation was inhibited by 50 μM Scutellarein treatment in 24 h and 48 h of treatment. The expression levels of phosphorylated EGFR, phosphorylated ERK, phosphorylated NFκB and COX-2 were reduced in a dose-dependent manner after 24 h Scutellarein treatments at different concentrations. Further, ERK inhibitor U0126 and NFκB inhibitor MG132 also inhibited A549 cell proliferation similar to 50 κM Scutellarein treatment from 24 h to 48 h.
CONCLUSIONS:
The experimental results showed that Scutellarein could inhibit proliferation of the human lung cancer cell line A549 through ERK and NFκB mediated by the EGFR pathway. |
|
| In vivo: |
| Pharmacol Biochem Behav. 2014 Mar;118:51-9. | | Neuroprotective effects of scutellarin and scutellarein on repeatedly cerebral ischemia-reperfusion in rats.[Pubmed: 24423938] | Scutellarin had protective effects against neuronal injury, however, there are few studies on the protective effect of Scutellarein, which is the main metabolite of scutellarin in vivo.
METHODS AND RESULTS:
This study investigated whether the neural injury by ischemia/reperfusion would be influenced by different doses of scutellarin and Scutellarein. Male Wistar rats were orally administered with scutellarin and Scutellarein at the doses of 0.09, 0.17, 0.35, 0.70, 1.40 mmol/kg, respectively; then after six consecutive days, they were subjected to global ischemia by occlusion of the bilateral common carotid arteries (BCCAO). After reperfusion for about 21 h, neurological and histological examinations were performed. The present results showed that Scutellarein attenuated neuronal cell damage, reduced cerebral water content, regulated the expression of glutamic acid (Glu), aspartic acid (Asp), glycine (Gly), γ-aminobutyric acid (GABA) and taurine (Tau), and improved the Ca(2+)-ATPase and Na(+),K(+)-ATPase activity. Meanwhile, significant difference was found among various doses of scutellarin and Scutellarein.
CONCLUSIONS:
Our studies indicated that scutellarin and Scutellarein could improve neuronal injury, and Scutellarein had better protective effect than scutellarin in rat cerebral ischemia. | | Int J Mol Med. 2015 Jan;35(1):31-8. | | Scutellarein inhibits cancer cell metastasis in vitro and attenuates the development of fibrosarcoma in vivo.[Pubmed: 25394920] | Fibrosarcoma is an aggressive and highly metastatic cancer of the connective tissue, for which effective therapeutic methods are limited. Recently, there has been a renewed interest in small molecular compounds from natural products in the treatment of cancer.
METHODS AND RESULTS:
In the present study, we investigated the compound, Scutellarein, extracted from the perennial herb Scutellaria lateriflora, and it was found to possess anticancer potential. Cell proliferation assay and cell cycle analysis revealed that the proliferation rate of HT1080 human fibrosarcoma cells was significantly suppressed by treatment with Scutellarein through the induction of apoptosis. Moreover, an in vivo experiment using Balb/c nude mice revealed that the volume and weight of the tumors were markedly reduced following treatment with Scutellarein. We also analyzed the effects of Scutellarein on the markers of metastasis, using the HT1080 cells. The results indicated that Scutellarein potently inhibited cell migration, invasion and the expression and activity of matrix metalloproteinase (MMP)-2, -9 and -14. Furthermore, MMP activation and cell survival were suppressed due to the Scutellarein-mediated downregulation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) activation.
CONCLUSIONS:
In conclusion, our data suggest that Scutellarein has the ability to attenuate the development of fibrosarcoma and inhibit cancer cell metastasis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)