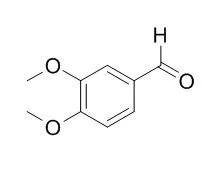
| Structure Identification: |
| Phys Chem Chem Phys. 2010 Jul 21;12(27):7603-11. | | Photoenhanced degradation of veratraldehyde upon the heterogeneous ozone reactions.[Pubmed: 20502834] | Light-induced heterogeneous reactions between gas-phase ozone and Veratraldehyde adsorbed on silica particles were performed.
METHODS AND RESULTS:
At an ozone mixing ratio of 250 ppb, the loss of Veratraldehyde largely increased from 1.81 x 10(-6) s(-1) in the dark to 2.54 x 10(-5) s(-1) upon exposure to simulated sunlight (lambda > 300 nm). The observed rates of degradation exhibited linear dependence with the ozone in the dark ozonolysis experiments which change in the non-linear Langmuir-Hinshelwood dependence in the experiments with simultaneous ozone and light exposure of the coated particles. When the coated silica particles were exposed only to simulated sunlight in absence of ozone the loss of Veratraldehyde was about three times higher i.e. 5.97 x 10(-6) s(-1) in comparison to the ozonolysis experiment under dark conditions at 250 ppb ozone mixing ratio, 1.81 x 10(-6) s(-1).These results clearly show that the most important loss of Veratraldehyde occurs under simultaneous ozone and light exposure of the coated silica particles. The main identified product in the heterogeneous reactions between gaseous ozone and adsorbed Veratraldehyde under dark conditions and in presence of light was veratric acid.Carbon yields of veratric acid were calculated and the obtained results indicated that at low ozone mixing ratio (250 ppb) the carbon yield obtained under dark conditions is 70% whereas the carbon yield obtained in the experiments with simultaneous ozone and light exposure of the coated particles is 40%. In both cases the carbon yield of veratric acid exponentially decayed leading to the plateau ( approximately 35% of carbon yield) at an ozone mixing ratio of 6 ppm.
CONCLUSIONS:
Two reaction products i.e. 3-hydroxy-4-methoxybenzoic acid and 4-hydroxy-3-methoxybenzoic acid were identified (confirmed with the standards) only in the experiments performed under simultaneous ozonolysis and light irradiation of the particles. | | Appl Environ Microbiol. 1994 Aug;60(8):2811-7. | | Anisaldehyde and Veratraldehyde Acting as Redox Cycling Agents for H(2)O(2) Production by Pleurotus eryngii.[Pubmed: 16349349] |
METHODS AND RESULTS:
The existence of a redox cycle leading to the production of hydrogen peroxide (H(2)O(2)) in the white rot fungus Pleurotus eryngii has been confirmed by incubations of 10-day-old mycelium with veratryl (3,4-dimethoxybenzyl) and anisyl (4-methoxybenzyl) compounds (alcohols, aldehydes, and acids). Veratraldehyde and anisaldehyde were reduced by aryl-alcohol dehydrogenase to their corresponding alcohols, which were oxidized by aryl-alcohol oxidase, producing H(2)O(2). Veratric and anisic acids were incorporated into the cycle after their reduction, which was catalyzed by aryl-aldehyde dehydrogenase. With the use of different initial concentrations of either veratryl alcohol, Veratraldehyde, or veratric acid (0.5 to 4.0 mM), around 94% of Veratraldehyde and 3% of veratryl alcohol (compared with initial concentrations) and trace amounts of veratric acid were found when equilibrium between reductive and oxidative activities had been reached, regardless of the initial compound used. At concentrations higher than 1 mM, veratric acid was not transformed, and at 1.0 mM, it produced a negative effect on the activities of aryl-alcohol oxidase and both dehydrogenases. H(2)O(2) levels were proportional to the initial concentrations of veratryl compounds (around 0.5%), and an equilibrium between aryl-alcohol oxidase and an unknown H(2)O(2)-reducing system kept these levels steady. On the other hand, the concomitant production of the three above-mentioned enzymes during the active growth phase of the fungus was demonstrated.
CONCLUSIONS:
Finally, the possibility that anisaldehyde is the metabolite produced by P. eryngii for the maintenance of this redox cycle is discussed. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)