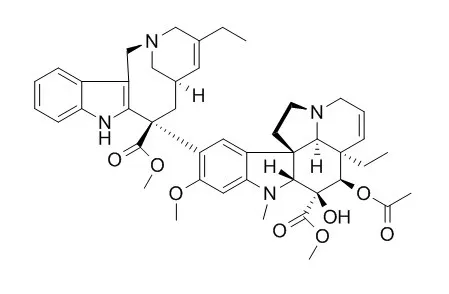
| Structure Identification: |
| AAPS PharmSciTech. 2014 Oct;15(5):1138-48. | | A new lipid-based nano formulation of vinorelbine.[Pubmed: 24871553] | Vinorelbine (VLB) is a semi-synthetic Vinca alkaloid which is currently used in treatment of different cancer types mainly advanced breast cancer (ABC) and advanced/metastatic non-small cell lung cancer (NSCLC). However, its marketed formulation has been reported to have serious side effects, such as granulocytopenia, which is the major dose-limiting toxicity. Other unwanted effects include venous discoloration and phlebitis proximal to the site of injection, as well as localized rashes and urticaria, blistering, and skin sloughing.
METHODS AND RESULTS:
Our long-term aim in synthesizing a novel nanomicellar Vinorelbine formulation is to reduce or even eliminate these side effects and increase drug activity by formulating the drug in a lipid-based system as a nanomedicine targeted to the site of action. To this end, the purpose of this study was to prepare, characterize, and determine the in vitro efficacy of Vinorelbine-loaded sterically stabilized, biocompatible, and biodegradable phospholipid nanomicelles (SSM; size, ∼15 nm). Our results indicated that Vinorelbine incorporate at high quantities and within the interface between the core and palisade sections of the micelles. Incorporation ratio of drug within sterically stabilized micelles increased as the total amount of drug in the system increased, and no drug particles were formed at the highest drug concentrations tested. The nanomicellar formulation of Vinorelbine was ∼6.7-fold more potent than Vinorelbine dissolved in DMSO on MCF-7 cell line.
CONCLUSIONS:
Collectively, these data indicate that Vinorelbine-loaded SSM can be developed as a new, safe, stable, and effective nanomedicine for the treatment of breast and lung cancers. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)