| In vitro: |
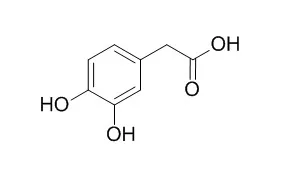
| Food Res Int. 2016 Nov;89(Pt 1):716-723. | | 3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides.[Pubmed: 28460970 ] | Since dietary flavonoid glycosides, including quercetin 4'-glucoside from onion, are poorly absorbed from the gastrointestinal tract, they are converted into smaller phenolic acids, which can be absorbed into the circulation. The purpose of this study was to compare the effects of the major phenolic acid catabolites of quercetin 4'-glucoside, including 3,4-Dihydroxyphenylacetic acid (DOPAC), 3-hydroxyphenylacetic acid, 3,4-dihydroxybenzoic acid (protocatechuic acid) and hippuric acid, on the antioxidant activity and phase II cytoprotective enzyme induction in vitro.
METHODS AND RESULTS:
Both DOPAC and protocatechuic acid, having a catechol moiety, exhibited both DPPH radical scavenging and superoxide dismutase-like activities, whereas 3-hydroxyphenyl acetic acid and hippuric acid did not. DOPAC also more potently enhanced the gene expression of several phase II drug-metabolizing enzymes than the other phenolic acid catabolites. DOPAC significantly inhibited the hydrogen peroxide-induced cytotoxicity in hepatocytes with the enhancement of the total glutathione S-transferase activity.
CONCLUSIONS:
In conclusion, DOPAC may play a key role in the antioxidative potential of the colonic lumen after the ingestion of the quercetin glycoside-rich onion. | | Biosci Biotechnol Biochem. 2017 Oct;81(10):1978-1983. | | 3,4-Dihydroxyphenylacetic acid is a potential aldehyde dehydrogenase inducer in murine hepatoma Hepa1c1c7 cells.[Pubmed: 28828965 ] | 3,4-Dihydroxyphenylacetic acid (DOPAC) is one of the major colonic microflora-produced catabolites of quercetin glycosides, such as quercetin 4'-glucoside derived from onion.
METHODS AND RESULTS:
Here, we investigated whether DOPAC modulates the aldehyde dehydrogenase (ALDH) activity and protects the cells from the acetaldehyde-induced cytotoxicity in vitro. DOPAC was shown to enhance not only the total ALDH activity, but also the gene expression of ALDH1A1, ALDH2 and ALDH3A1 in a concentration-dependent manner. DOPAC simultaneously stimulated the nuclear translocation of NFE2-related factor 2 and aryl hydrocarbon receptor. The pretreatment of DOPAC completely protected the cells from the acetaldehyde-induced cytotoxicity.
CONCLUSIONS:
The present study suggested that DOPAC acts as a potential ALDH inducer to prevent the alcohol-induced abnormal reaction. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)