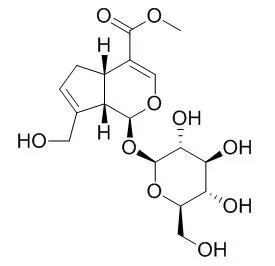
| Description: |
Geniposide exhibits anti-diabetic, antidepressant-like, antioxidative, anti-apoptotic, antiproliferative and neuroprotective activities. Geniposide is an agonist for GLP-1 receptor, it regulates expression of anti-oxidative proteins including HO-1 and Bcl-2 by activating the transcriptor of p90RSK via MAPK signaling pathway in PC12 cells. Geniposide may suppress TGF-β1-induced EMT in hepatic fibrosis by inhibiting the TGFβ/Smad and ERK-mitogen-activated protein kinase (MAPK) signaling pathways. |
| In vitro: |
| Neurochem Int. 2007 Nov-Dec;51(6-7):361-9. | | Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway.[Pubmed: 17629357] | Alzheimer's disease (AD) is the most common form of dementia. Glucagon-like peptide-1 (GLP-1) gives a new genre in therapeutic targets for intervention in AD with its neurotrophic and neuroprotective functions. In previous work, we identified that Geniposide is a novel agonist for GLP-1 receptor, which shows neurotrophic characteristics to induce the neuronal differentiation of PC12 cells.
METHODS AND RESULTS:
The aim of this study is to determine whether Geniposide prevents neurons from oxidative damage, and to explore its signaling pathways. The results demonstrated that Geniposide increased the expression of anti-apoptotic proteins, including Bcl-2 and heme oxygenase-1 (HO-1), to antagonize the oxidative damage in PC12 cells induced by hydrogen peroxide. LY294002 (a PI3K inhibitor) inhibited the effect of Geniposide increasing of Bcl-2 level by activation of MAPK, MEK and c-Raf phosphorylation in hydrogen peroxide treated PC12 cells. U0126 (a selective inhibitor of MEK) also attenuated the enhancement of Geniposide on Bcl-2 level by inhibiting the phosphorylation of p90RSK in the hydrogen peroxide treated PC12 cells.
CONCLUSIONS:
All these data demonstrate that Geniposide, an agonist for GLP-1 receptor, regulates expression of anti-oxidative proteins including HO-1 and Bcl-2 by activating the transcriptor of p90RSK via MAPK signaling pathway in PC12 cells. | | Int Immunopharmacol. 2012 Dec;14(4):792-8. | | Geniposide, from Gardenia jasminoides Ellis, inhibits the inflammatory response in the primary mouse macrophages and mouse models.[Pubmed: 22878137 ] | Geniposide, a main iridoid glucoside component of gardenia fruit, has been known to exhibit antibacterial, anti-inflammatory and other important therapeutic activities. The objective of this study was to investigate the protective effects of Geniposide on inflammation in lipopolysaccharide (LPS) stimulated primary mouse macrophages in vitro and LPS induced lung injury model in vivo.
METHODS AND RESULTS:
The expression of pro-inflammatory cytokines was determined by enzyme-linked immunosorbent assay (ELISA). Nuclear factor-kappa B (NF-κB), inhibitory kappa B (IκBα) protein, p38, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and Toll-like receptor 4 (TLR4) were determined by Western blot. Further analysis was carried out in mTLR4 and mMD-2 co-transfected HEK293 cells. The results showed that Geniposide markedly inhibited the LPS-induced TNF-α, IL-6 and IL-1β production both in vitro and in vivo. Geniposide blocked the phosphorylation of IκBα, p65, p38, ERK and JNK in LPS stimulated primary mouse macrophages. Furthermore, Geniposide inhibited the expression of TLR4 in LPS stimulated primary mouse macrophages and inhibited the LPS-induced IL-8 production in HEK293-mTLR4/MD-2 cells. In vivo study, it was also observed that Geniposide attenuated lung histopathologic changes in the mouse models.
CONCLUSIONS:
These results suggest that Geniposide exerts an anti-inflammatory property by down-regulating the expression of TLR4 up-regulated by LPS. Geniposide is highly effective in inhibiting acute lung injury and may be a promising potential therapeutic reagent for acute lung injury treatment. | | Ethnopharmacol . 2016 Jun 5;185:77-86. | | Geniposide attenuates inflammatory response by suppressing P2Y14 receptor and downstream ERK1/2 signaling pathway in oxygen and glucose deprivation-induced brain microvascular endothelial cells[Pubmed: 26976766] | | Ethnopharmacological relevance: Fructus gardenia is widely used for treatment of stroke and infectious diseases in Chinese medicine. Geniposide is the key bioactive compound related to the pharmacodynamic actions of gardenia on ischemic stroke. The molecular mechanism by which Geniposide improves the ischemic brain injury was observed in the study.
Aim of the study: Recent studies showed that Geniposide had protective activities against the inflammatory response in ischemic stroke. However, the molecular mechanism of Geniposide anti-inflammatory role has not yet been fully elucidated. In this study, we investigated the effect of Geniposide on the expression of P2Y14 receptor and downstream signaling pathway in brain microvascular endothelial cells (BMECs).
Materials and methods: An in vitro model of cerebral ischemia in BMECs was established by oxygen-glucose-deprivation (OGD). To further confirm the specific effect of Geniposide on P2Y14 receptor and downstream signaling pathways, we set up a UDP-glucose (an agonist of the P2Y14 receptor) stimulated model. After administration of Geniposide, the expression of P2Y14 receptor, phosphorylation of RAF-1, mitogen activated protein kinase kinase1/2 (MEK1/2), extracellular signal-regulated kinase 1/2 (ERK1/2), level of interleukin-8 (IL-8), interleukin-1β (IL-1β), monocyte chemotactic protein 1 (MCP-1) in BMECs were determined.
Results: The mRNA and protein expression of P2Y14 in the rat BMECs were up-regulated in OGD-induced injury. After administration of Geniposide, the expression of P2Y14 receptor was significantly down-regulated, the phosphorylation of RAF-1, MEK1/2, ERK1/2 were suppressed. Similar data were obtained in UDP-glc stimulated model. We also observed that Geniposide markedly declined the production of IL-8, IL-1β and MCP-1 in OGD-induced BMECs.
Conclusion: Geniposide exerted anti-inflammatory effects by interfering with the expression of P2Y14 receptor, which subsequently inhibits the downstream ERK1/2 signaling pathways and the release of the pro-inflammatory cytokines IL-8, MCP-1, IL-1β. Therefore, this study provides the evidence for gardenia's clinical application in cerebral ischemia.
Keywords: Anti-inflammation; Brain microvascular endothelial cells; ERK1/2 signaling pathway; Geniposide; Geniposide (PubChem CID: 107848); P2Y(14) receptor.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved. |
|
| In vivo: |
| Eur Neuropsychopharmacol. 2015 Apr 17. | | Antidepressant-like effect of geniposide on chronic unpredictable mild stress-induced depressive rats by regulating the hypothalamus-pituitary-adrenal axis.[Pubmed: 25914157] | Geniposide as the major active component of Gardenia jasminoides Ellis has neuroprotective activity.
METHODS AND RESULTS:
This study elucidated the potential antidepressant-like effect of Geniposide and its related mechanisms using a depression rat model induced by 3 consecutive weeks of chronic unpredictable mild stress (CUMS). Sucrose preference test, open field test (OFT) and forced swimming test (FST) were applied to evaluate the antidepressant effect of Geniposide. We found that Geniposide (25, 50, 100mg/kg) treatment reversed the CUMS-induced behavioral abnormalities, as suggested by increased sucrose intake, improved crossing and rearing behavior in OFT, shortened immobility and prolonged swimming time in FST. Additionally, Geniposide treatment normalized the CUMS-induced hyperactivity of HPA axis, as evidenced by reduced CORT serum level, adrenal gland index and hypothalamic CRH mRNA expression, with no significant effect on ACTH serum level. Moreover, Geniposide treatment upregulated the hypothalamic GRα mRNA level and GRα protein expression in PVN, suggesting Geniposide could recover the impaired GRα negative feedback on CRH expression and HPA axis.
CONCLUSIONS:
These aforementioned therapeutic effects of Geniposide were essentially similar to fluoxetine.
Our results indicated that Geniposide possessed potent antidepressant-like properties that may be mediated by its effects on the HPA axis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)