| Kinase Assay: |
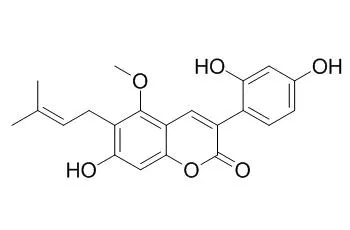
| Eur J Pharm Sci. 2017 Nov 15;109:182-190. | | Cytochrome P450 inhibition by three licorice species and fourteen licorice constituents.[Pubmed: 28774812] |
METHODS AND RESULTS:
The potential of licorice dietary supplements to interact with drug metabolism was evaluated by testing extracts of three botanically identified licorice species (Glycyrrhiza glabra L., Glycyrrhiza uralensis Fish. ex DC. and Glycyrrhiza inflata Batalin) and 14 isolated licorice compounds for inhibition of 9 cytochrome P450 enzymes using a UHPLC-MS/MS cocktail assay. G. glabra showed moderate inhibitory effects against CYP2B6, CYP2C8, CYP2C9, and CYP2C19, and weak inhibition against CYP3A4 (testosterone). In contrast, G. uralensis strongly inhibited CYP2B6 and moderately inhibited CYP2C8, CYP2C9 and CYP2C19, and G. inflata strongly inhibited CYP2C enzymes and moderately inhibited CYP1A2, CYP2B6, CYP2D6, and CYP3A4 (midazolam). The licorice compounds isoliquiritigenin, licoricidin, licochalcone A, 18β-glycyrrhetinic acid, and Glycycoumarin inhibited one or more members of the CYP2C family of enzymes. Glycycoumarin and licochalcone A inhibited CYP1A2, but only Glycycoumarin inhibited CYP2B6. Isoliquiritigenin, glabridin and licoricidin competitively inhibited CYP3A4, while licochalcone A (specific to G. inflata roots) was a mechanism-based inhibitor. The three licorice species commonly used in botanical dietary supplements have varying potential for drug-botanical interactions as inhibitors of cytochrome P450 isoforms.
CONCLUSIONS:
Each species of licorice displays a unique profile of constituents with potential for drug interactions. | | Steroids. 2016 Jan;105:42-9. | | Licorice root components in dietary supplements are selective estrogen receptor modulators with a spectrum of estrogenic and anti-estrogenic activities.[Pubmed: 26631549 ] | Licorice root extracts are often consumed as botanical dietary supplements by menopausal women as a natural alternative to pharmaceutical hormone replacement therapy. In addition to their components liquiritigenin (Liq) and isoliquiritigenin (Iso-Liq), known to have estrogenic activity, licorice root extracts also contain a number of other flavonoids, isoflavonoids, and chalcones.
METHODS AND RESULTS:
We have investigated the estrogenic activity of 7 of these components, obtained from an extract of Glycyrrhiza glabra powder, namely Glabridin (L1), Calycosin (L2), Methoxychalcone (L3), Vestitol (L4), Glyasperin C (L5), Glycycoumarin (L6), and Glicoricone (L7), and compared them with Liq, Iso-Liq, and estradiol (E2). All components, including Liq and Iso-Liq, have low binding affinity for estrogen receptors (ERs). Their potency and efficacy in stimulating the expression of estrogen-regulated genes reveal that Liq and Iso-Liq and L2, L3, L4, and L6 are estrogen agonists. Interestingly, L3 and L4 have an efficacy nearly equivalent to E2 but with a potency ca. 10,000-fold less. The other components, L1, L5 and L7, acted as partial estrogen antagonists. All agonist activities were reversed by the antiestrogen, ICI 182,780, or by knockdown of ERα with siRNA, indicating that they are ER dependent. In HepG2 hepatoma cells stably expressing ERα, only Liq, Iso-Liq, and L3 stimulated estrogen-regulated gene expression, and in all cases gene stimulation did not occur in HepG2 cells lacking ERα.
CONCLUSIONS:
Collectively, these findings classify the components of licorice root extracts as low potency, mixed ER agonists and antagonists, having a character akin to that of selective estrogen receptor modulators or SERMs. | | J Ethnopharmacol. 2006 May 24;105(3):409-14. | | Glycycoumarin from Glycyrrhizae Radix acts as a potent antispasmodic through inhibition of phosphodiesterase 3.[Pubmed: 16387459 ] | Glycyrrhizae Radix is used to treat abdominal pain as a component of Shakuyaku-kanzo-to, a traditional Chinese medicine formulation. We aim at clarifying the antispasmodic principles of Glycyrrhizae Radix, and consequently isolated Glycycoumarin as a potent relaxant on the carbamylcholine (CCh)-induced contraction of mouse jejunum.
METHODS AND RESULTS:
In this paper we investigated the effects and the action mechanism of Glycycoumarin on the contraction of mouse jejunum. Glycycoumarin inhibited the contraction induced by various types of stimulants, such as CCh, KCl, BaCl(2), and A23187 (calcium ionophore III) with IC(50) values of 2.93+/-0.94 micromol/l (1.08+/-0.35 microg/ml), 2.59+/-0.58 micromol/l (0.95+/-0.29 microg/ml), 4.09+/-1.82 micromol/l (1.51+/-0.67 microg/ml) and 7.39+/-5.19 micromol/l (2.72+/-1.91 microg/ml), respectively, with a potency similar to that of papaverine (a representative antispasmodic for smooth muscle). Furthermore, pretreatment with Glycycoumarin enhanced the relaxation induced by forskolin on CCh-evoked contraction, similar to that by pretreatment with IBMX, a non-specific inhibitor of phosphodiesterases (PDEs). Pretreatment with Glycycoumarin also enhanced the relaxation effect of rolipram, a specific inhibitor of PDE isozyme 4, as pretreatment with milrinone, a specific inhibitor of isozyme 3, did. Moreover, the effect of Glycycoumarin was associated with dose-dependent accumulation of cAMP, but not cGMP, in mouse jejunum.
CONCLUSIONS:
These results indicate that Glycycoumarin has an inhibitory effect on smooth muscle contraction induced by various types of stimulants through the inhibition of PDEs, especially isozyme 3, followed by the accumulation of intracellular cAMP. |
|
| Cell Research: |
| Sci Rep. 2016 Nov 30;6:38138. | | Glycycoumarin inhibits hepatocyte lipoapoptosis through activation of autophagy and inhibition of ER stress/GSK-3-mediated mitochondrial pathway.[Pubmed: 27901086 ] | Herbal medicine as an alternative approach in the treatment of disease has drawn growing attention. Identification of the active ingredient is needed for effective utilization of the herbal medicine. Licorice is a popular herbal plant that is widely used to treat various diseases including liver diseases. Glycycoumarin (GCM) is a representative of courmarin compounds isolated from licorice.
METHODS AND RESULTS:
In the present study, the protective effect of GCM on hepatocyte lipoapoptosis has been evaluated using both cell culture model of palmitate-induced lipoapoptosis and animal model of non-alcoholic steatohepatitis (NASH). The results demonstrated for the first time that GCM was highly effective in suppressing hepatocyte lipoapoptosis in both in vitro and in vivo. Mechanistically, GCM was able to re-activate the impaired autophagy by lipid metabolic disorders. In line with the activation of autophagy, ER stress-mediated JNK and mitochondrial apoptotic pathway activation was inhibited by GCM both in vitro and in vivo. In addition, inactivation of GSK-3 might also contribute to the protective effect of GCM on hepatocyte lipoapoptosis.
CONCLUSIONS:
Our findings supported GCM as a novel active component of licorice against non-alcoholic fatty liver disease (NAFLD). | | J Ethnopharmacol. 2015 Jan 15;159:122-8. | | Protective effects of glycycoumarin and procyanidin B1, active components of traditional Japanese medicine yokukansan, on amyloid β oligomer-induced neuronal death.[Pubmed: 25446602 ] | Yokukansan, a traditional Japanese (Kampo) medicine, is composed of seven medicinal herbs, and has been traditionally used to treat neurosis, insomnia, and night crying and irritability in children. Yokukansan and its constituent herbs, Glycyrrhiza and Uncaria Hook, have recently been shown to have protective effects against amyloid β (Aβ) oligomer-induced apoptosis by suppressing the activation of caspase-3 in primary cultured neurons. The aim of the present study was to identify the effective components of Glycyrrhiza and Uncaria Hook against Aβ oligomer-induced neurotoxicity. We also attempted to clarify the mechanisms by which yokukansan and these herbs, as well as their components, suppressed the activation of caspase-3 in Aβ oligomer-treated neurons.
METHODS AND RESULTS:
Rat primary cultured cortical neurons were treated with Aβ oligomer (3 μM). The protective effects of 16 components derived from Glycyrrhiza or Uncaria Hook against Aβ oligomer-induced neurotoxicity were determined using the MTT reduction assay 48 h after the treatment. The suppressive effects of the test substances, i.e., yokukansan, Glycyrrhiza, Uncaria Hook, and screened components, on the Aβ oligomer-induced activation of caspase-3(/7) were evaluated using the caspase-Glo assay 48 h after the Aβ oligomer treatment. The suppressive effects of the test substances on the activation of caspase-8 and -9, both of which are located upstream of caspase-3, were also examined 24h after the Aβ oligomer treatment.
Two of the 16 components tested, Glycycoumarin derived from Glycyrrhiza and procyanidin B1 derived from Uncaria Hook, significantly inhibited Aβ oligomer-induced neuronal death in a dose-dependent manner. Glycyrrhiza, Uncaria Hook, and yokukansan significantly suppressed the Aβ oligomer-induced activation of caspase-3 as well as caspase-8 and -9. Glycycoumarin also suppressed the activation of caspase-3, but not caspase-8 and -9. Procyanidin B1 suppressed the activation of caspase-3, -8, and -9.
CONCLUSIONS:
Our results demonstrated that Glycycoumarin and procyanidin B1 had ameliorative effects on Aβ oligomer-induced neurotoxicity. The neuroprotective effects of Glycycoumarin are thought to be due to the attenuated activation of caspase-3, but not caspase-8 or -9. Procyanidin B1, as well as yokukansan, Glycyrrhiza, and Uncaria Hook, may attenuate the activation of caspase-3 by inhibiting that of caspase-8 and -9. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)