| Structure Identification: |
| Phytochemistry. 2016 Dec;132:76-85. | | Toxic aromatic compounds from fruits of Narthecium ossifragum L.[Pubmed: 27720435 ] | The intake of Narthecium ossifragum, commonly known as bog asphodel, has been associated with toxic effects observed in sheep for centuries. Although the plant has been studied for five centuries little is known about its chemical constituents.
METHODS AND RESULTS:
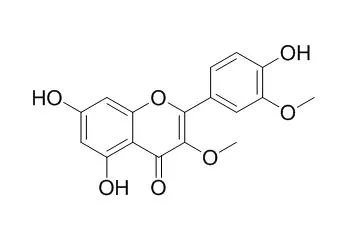
Six previously undescribed natural products, naringenin(3 → 6″)luteolin, naringenin(3 → 6″)chrysoeriol, liovil 4-O-β-glucopyranoside, 2,6-dimethoxy cinnamic acid, (E)-4-(3-hydroxy-2,2-dimethylchroman-6-yl)but-3-en-2-one and (E)-4-(4-(((E)-4-hydroxy-3-methylbut-2-en-1-yl)oxy)phenyl)but-3-en-2-one, have been identified from fruits of N. ossifragum for the first time. In addition, the rare natural product 4-hydroxy-3-(3-methylbut-2-enyl)benzaldehyde and the five known compounds 4-hydroxycinnamic acid, Quercetin 3,3'-dimethyl ether, quercetin 3,7-dimethyl ether, chrysoeriol 7-O-β-glucopyranoside and the di-C-glycosylflavone isoschaftoside were all characterized for the first time from the fruits of N. ossifragum.
CONCLUSIONS:
The discovery of sufficient amounts of 4-hydroxy-3-(3-methylbut-2-enyl)benzaldehyde in fresh plant material of N. ossifragum to allow complete structure elucidation by NMR and HRMS supports the possibility that fungi associated with N. ossifragum may be able to produce enough toxins to play a significant role in the pathogenicity of N. ossifragum. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)