| Kinase Assay: |
| Bioorg Med Chem Lett. 2011 Dec 1;21(23):7041-4. | | Ungeremine effectively targets mammalian as well as bacterial type I and type II topoisomerases.[Pubmed: 22014547] |
METHODS AND RESULTS:
From the methanol extract of the bulbs of Pancratium illyricum L., three phenanthridine type alkaloids, Ungeremine (1), (-)-lycorine (2) and (+)-vittatine (3) were isolated. For the evaluation of their anticancer and antibacterial potential, compounds 1-3 were tested against human (I, IIα) and bacterial (IA, IV) topoisomerases.
CONCLUSIONS:
Our data demonstrated that Ungeremine impairs the activity of both, human and bacterial topoisomerases. Remarkably, Ungeremine was found to largely increments the DNA cleavage promoted by bacterial topoisomerase IA, a new target in antimicrobial chemotherapy.. |
|
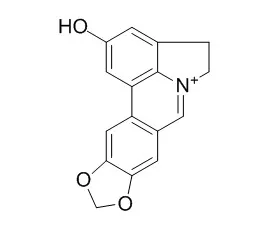
| Structure Identification: |
| J Agric Food Chem. 2013 Feb 13;61(6):1179-83. | | Ungeremine and Its hemisynthesized analogues as bactericides against Flavobacterium columnare.[Pubmed: 23331165 ] | The Gram-negative bacterium Flavobacterium columnare is the cause of columnaris disease, which can occur in channel catfish ( Ictalurus punctatus ). In a previous study, the betaine-type alkaloid Ungeremine, 1, obtained from Pancratium maritimum L. was found to have strong antibacterial activity against F. columnare.
METHODS AND RESULTS:
In this study, analogues of 1 were evaluated using a rapid bioassay for activity against F. columnare to determine if the analogues might provide greater antibacterial activity and to determine structure-activity relationships of the test compounds. Several Ungeremine analogues were prepared by hydrochlorination of the alkaloid and by selenium dioxide oxidation of both lycorine, 7, and pseudolycorine, 8, which yielded the isomer of Ungeremine, 3, and zefbetaine, 4, respectively. The treatment of lycorine with phosphorus oxychloride allowed the synthesis of an anhydrolycorine lactam, 5, showing, with respect to 1, the deoxygenation and oxygenation of C-2 and C-7 of the C and B rings, respectively.
CONCLUSIONS:
The results of the structure-activity relationship studies showed that the aromatization of the C ring and the oxidation to an azomethine group of C-7 of the B ring are structural features important for antibacterial activity. In addition, the position of the oxygenation of the C ring as well as the presence of the 1,3-dioxole ring joined to the A ring of the pyrrolo[de]phenanthridine skeleton also plays a significant role in imparting antibacterial activity. On the basis of 24-h 50% inhibition concentration (IC(50)) results, Ungeremine hydrochloride, 2, was similar in toxicity to 1, whereas 5 had the lowest activity. Analogue 2 is soluble in water, which may provide the benefit for use as an effective feed additive or therapeutant compared to Ungeremine. | | Biol Pharm Bull. 2004 Nov;27(11):1804-9. | | Isolation of the acetylcholinesterase inhibitor ungeremine from Nerine bowdenii by preparative HPLC coupled on-line to a flow assay system.[Pubmed: 15516727] | In an attempt to isolate the active compound while detecting acetylcholinesterase inhibitory activity, we applied a fluorometric flow assay system to an on-line coupled preparative HPLC.
METHODS AND RESULTS:
The MeOH extract of Nerine bowdenii showed a strong inhibitory peak in the on-line assay, and the active compound was isolated by CPC and HPLC. It was identified as Ungeremine by analysis of its (1)H-NMR, 2D-NMR, and NOESY spectra. The assignment of the active N. bowdenii constituent was also confirmed by co-TLC, co-HPLC, and co-(1)H-NMR experiments using an authentic sample of synthetic Ungeremine.
CONCLUSIONS:
The IC(50) value of Ungeremine was 0.35 microM, showing stronger activity than galanthamine (2.2 microM). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)