| In vitro: |
| Geochimica Et Cosmochimica Acta, 2003, 67(19):3645-3664. | | Experimental study of the hydrothermal reactivity of organic acids and acid anions: II. Acetic acid, acetate, and valeric acid[Reference: WebLink] |
Organic acids and acid anions occur in substantial concentrations in many aqueous geologic fluids and are thought to take part in a variety of geochemical processes ranging from the transport of metals in ore-forming fluids to the formation of natural gas to serving as a metabolic energy source for microbes in subsurface habitats.
The widespread occurrence of organic acids and their potential role in diverse geologic processes has led to numerous experimental studies of their thermal stability, yet there remain substantial gaps in our knowledge of the factors that control the rates and reaction pathways for the decomposition of these compounds under geologic conditions.
METHODS AND RESULTS:
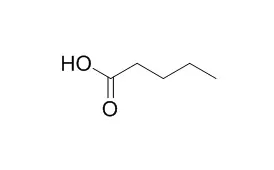
In order to address some of these uncertainties, a series of laboratory experiments were conducted to examine the behavior of organic acids and acid anions under hydrothermal conditions in the presence of minerals. Reported here are results of experiments where aqueous solutions of acetic acid, sodium acetate, or Valeric acid (n-pentanoic acid) were heated at 325°C, 350 bars in the presence of the mineral assemblages hematite + magnetite + pyrite, pyrite + pyrrhotite + magnetite, and hematite + magnetite. The results indicate that aqueous acetic acid and acetate decompose by a combination of two reaction pathways: decarboxylation and oxidation. Both reactions are promoted by minerals, with hematite catalyzing the oxidation reaction while magnetite catalyzes decarboxylation. The oxidation reaction is much faster, so that oxidation dominates the decomposition of acetic acid and acetate when hematite is present. In contrast to previous reports that acetate decomposed more slowly than acetic acid, we found that acetate decomposed at slightly faster rates than the acid in the presence of minerals. Although longer-chain monocarboxylic acids are generally thought to decompose by decarboxylation, Valeric acid appeared to decompose primarily by “deformylation” to 1-butene plus formic acid. Subsequent decomposition of 1-butene and formic acid generated a variety of short-chain (≤C4) hydrocarbons and moncarboxylic acids as well as CO2. Valeric acid decomposition proceeded more rapidly (by a factor of 2) in the presence of hematite-magnetite-pyrite than with the other mineral assemblages, with the greater reaction rate apparently attributable to the effects of fluid chemistry. Valeric acid was observed to decompose at a substantially faster rate than acetic acid under similar conditions.
CONCLUSIONS:
The results suggest that decomposition of aqueous monocarboxylic acids may make a significant contribution to the conversion of petroleum to light hydrocarbons in natural gas and thermal fluids. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)