| Structure Identification: |
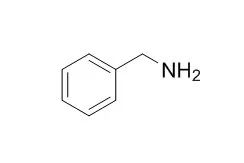
| Bioorg Med Chem. 2014 Mar 1;22(5):1558-67. | | Synthesis of 2,6-disubstituted benzylamine derivatives as reversible selective inhibitors of copper amine oxidases.[Pubmed: 24529308] | In order to obtain substrate-like inhibitors of copper amine oxidases (CAOs), a class of enzymes involved in important cellular processes as well as in crosslinking of elastin and collagen and removal of biogenic primary amines, we synthesized a set of Benzylamine derivatives properly substituted at positions 2 and 6 and studied their biological activity towards some members of CAOs.
METHODS AND RESULTS:
With Benzylamines 6, 7, 8 containing linear alkoxy groups we obtained reversible inhibitors of Benzylamine oxidase (BAO), very active and selective toward diamine oxidase (DAO), lysyl oxidase (LO) and monoamine oxidase B (MAO B) characterized by a certain toxicity consequent to the crossing of the brain barrier. Poorly toxic, up to very active, reversible inhibitors of BAO, very selective toward DAO, LO and MAO B, were obtained with Benzylamines 10, 11, 12 containing hydrophilic ω-hydroxyalkoxy groups. With Benzylamines 13, 14, 15, containing linear alkyl groups endowed with steric, but not conjugative effects for the absence of properly positioned oxygen atoms, we synthesized moderately active inhibitors of BAO reversible and selective toward DAO, LO and MAO B. The cross examination of the entire biological data brought us to the conclusion that the bioactive synthesized compounds most likely exert their physiological role of reversible inhibitors in consequence of the formation of a plurality of hydrogen bonds or hydrophobic non-covalent interactions with proper sites in the protein.
CONCLUSIONS:
Accordingly, the reported inhibitors may be considered as a set of research tools for general biological studies and the formation of enzyme complexes useful for X-ray structure determinations aimed at the design of more sophisticated inhibitors to always better modulate the protein activity without important side effects. | | Spectrochim Acta A Mol Biomol Spectrosc. 2014 May 5;125:297-307. | | Spectroscopic, crystallographic and theoretical studies of lasalocid complex with ammonia and benzylamine.[Pubmed: 24562161] | A natural antibiotic--Lasalocid is able to form stable complexes with ammonia and organic amines.
METHODS AND RESULTS:
New complexes of lasalocid with Benzylamine and ammonia were obtained in the crystal forms and studied using X-ray, FT-IR, (1)H NMR, (13)C NMR and DFT methods. These studies have shown that in both complexes the proton is transferred from the carboxylic group to the amine group with the formation of a pseudo-cyclic structure of lasalocid anion complexing the protonated amine or NH4(+) cation.
CONCLUSIONS:
The spectroscopic and DFT studies demonstrated that the structure of the complex formed between Lasalocid and Benzylamine in the solid is also conserved in the solution and gas phase. In contrast, the structure of the complex formed between lasalocid and ammonium cation found in the solid state undergoes dissociation in chloroform solution accompanied with a change in the coordination form of the NH4(+) cation. | | J Enzyme Inhib Med Chem. 2014 Apr;29(2):168-74. | | Synthesis and carbonic anhydrase isoenzymes I and II inhibitory effects of novel benzylamine derivatives.[Pubmed: 23391138] | Synthesis and carbonic anhydrase inhibitory properties of novel diarylmethylamines 22-25 and sulfonamide derivatives 26-28 were investigated.
METHODS AND RESULTS:
Acylation of methoxy-substituted benzenes with benzene carboxylic acids, reduction of ketones with NaBH4, conversion of alcohols to azides, Pd-C catalyzed hydrogenation of azides afforded title compounds 22-25. Compounds 22, 24 and 25 were converted to sulfonamide derivatives 26-28 with MeSO2Cl. The inhibitory effects of novel Benzylamine derivatives 22-28 were tested on human carbonic anhydrase (hCA, EC 4.2.1.1) isozymes hCA I and II.
CONCLUSIONS:
The results demonstrated that compound 28 was found to be the best inhibitor against both hCA I (Ki: 3.68 µM) and hCA II (Ki: 9.23 µM). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)