| Kinase Assay: |
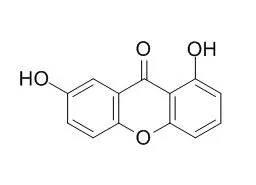
| Phytomedicine. 2010 Jul;17(8-9):690-2. | | Mechanism of the vasodilator effect of Euxanthone in rat small mesenteric arteries.[Pubmed: 20097048] | In the present work we investigated the mechanism involved in the vasodilator effect induced by Euxanthone in rat small mesenteric arteries.
METHODS AND RESULTS:
We observed that Euxanthone induced concentration-dependent vasodilatation in arteries by a mechanism independent on the release of endothelial factors, such as nitric oxide (NO) and cyclooxygenase-derived factors. In addition our results also suggest that Euxanthone induced its vasodilator effect through inhibition of calcium-sensitive mechanisms activated by protein kinase C, rather than by inhibition of contractions dependent on the release of the intracellular calcium stores or by inhibition of voltage-operated calcium channels. | | Int J Biochem Cell Biol. 2006;38(8):1393-401. | | Involvement of protein kinase C and E2F-5 in euxanthone-induced neurite differentiation of neuroblastoma.[Pubmed: 16546434] | Euxanthone, a neuritogenic agent isolated from the medicinal herb Polygala caudata, has been shown to induce morphological differentiation and neurite outgrowth in murine neuroblastoma Neuro 2a cells (BU-1 subclone). In order to elucidate the underlying mechanisms of Euxanthone-induced neurite outgrowth, a proteomic approach was employed.
METHODS AND RESULTS:
In the present study, two dimensional (2-D) gel electrophoresis and matrix-assisted laser desorption/ionization-time of flight (MALDI-ToF) mass spectrometry were performed to investigate the alterations in protein expression profile of Euxanthone-treated BU-1 cells. Fourteen identified proteins were changed in expression levels after induction of neurite growth. These proteins included participants in transcription and cell cycle regulation, calcium influx and calcium signaling, fatty acid metabolism, cytoskeleton reorganization, casein kinase signal transduction, putative transbilayer amphipath transport and protein biosynthesis. Among the 14 identified proteins, E2F transcription factor 5 (E2F-5) was significantly up-regulated after Euxanthone treatment. Go6976, a protein kinase C (PKC) alpha/betaI inhibitor, was found to inhibit neuritogenesis and expression of E2F-5 in the Euxanthone-treated BU-1 cells, while SH-6, the Akt/PKB inhibitor, had no inhibitory effect. The gene silencing of E2F-5 by small interfering RNA (siRNA) was found to abolish the Euxanthone-induced neurite outgrowth.
CONCLUSIONS:
In conclusion, these results indicated that the transcription factor E2F-5 was actively involved in the regulation of Euxanthone-induced neurite outgrowth via PKC pathway. | | Planta Med. 2002 Nov;68(11):1039-41. | | Differential activation of protein kinase C isoforms by euxanthone, revealed by an in vivo yeast phenotypic assay.[Pubmed: 12451499] |
METHODS AND RESULTS:
The protein kinase C (PKC) modulatory effects of Euxanthone, isolated from the wood of Cratoxylum maingayi, on isoforms alpha, betaI, delta, eta and zeta were characterised using an alternative in vivo yeast phenotypic assay. The present study shows that Euxanthone can activate isoforms alpha, betaI, delta, eta and zeta, being more effective on PKC-betaI, -delta, -eta and -zeta than the established PKC activators used (the phorbol ester PMA and arachidonic acid for PKC-zeta). Furthermore, Euxanthone presents differences on its potency towards individual PKC isoforms, showing a remarkable selectivity for PKC-zeta.
CONCLUSIONS:
These results can help to clarify the molecular basis of the Euxanthone-mediated effects. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)