| In vitro: |
| Immunopharmacol Immunotoxicol. 2011 Dec;33(4):663-6. | | Antiplasmodial and cytotoxic activity of coumarin derivatives from dried roots of Angelica gigas Nakai in vitro.[Pubmed: 21428713] | The butanol-soluble fraction of the dried root of Angelica gigas exhibited significant protection against chloroquine-sensitive strains of Plasmodium falciparum using the parasite lactate dehydrogenase assay method.
METHODS AND RESULTS:
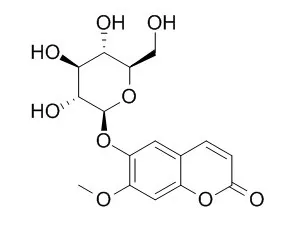
Using antiplasmodial activity-guided fractionation, five coumarins, marmesinin (1), nodakenin (2), skimmin (3), apiosylskimmin (4), and Magnolioside (5), were isolated and evaluated for in vitro antiplasmodial activity, as well as for their cytotoxic potential on SK-OV-3 cancer cell lines. Compounds 1 and 5 showed notable growth inhibitory activity against chloroquine-sensitive strains of P. falciparum with IC(50) values of 5.3 and 8.2 μM. The compounds showed no significant cytotoxicity (IC(50) > 100 μM) toward the SK-OV-3 cancer cell line.
CONCLUSIONS:
This is the first report on the antiplasmodial activity of these coumarin derivatives from the dried root of A. gigas. | | Phytochemistry Letters, 2016 , 18 :23-28. | | Chemical composition, antibacterial, antioxidant and tyrosinase inhibitory activities of glycosides from aerial parts of Eryngium tricuspidatum L.[Reference: WebLink] | Two new phenolic glucosides, together with six known compounds, were isolated from the aerial part of Eryngium tricuspidatum L. (Apiaceae).
METHODS AND RESULTS:
The structures of the new compounds were established as 2-hydroxy- 3,5-dimethyl-acetophenon-4-O-β-d-glucopyranoside (1) and 2,3-dimethyl-4-hydroxymethylphenyl-1-hydroxymethyl-O-β-d-glucopyranoside (2) on the basis of detailed spectroscopic data including MS, 1D, and 2D NMR. The antibacterial, tyrosinase inhibitory and DPPH radical scavenging activities of hydromethanolic extract, fractions, and the eight isolated compounds were evaluated. The antibacterial assay showed a moderate activity for Magnolioside (4) against Staphylococcus aureus CIP 53.154. Compound 7 (quercetin 3-O-β-d-glucopyranosyl-(1 → 6)-O-β-d-galactopyranoside) had moderate DPPH radical scavenging activity whereas compounds 2 exhibited good inhibitory effect against mushroom tyrosinase. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)