| Structure Identification: |
| Yao Xue Xue Bao. 2006 Apr;41(4):380-4. | | [Qualitative and quantitative determination of the main components of huanglianjiedu decoction by HPLC-UV/MS].[Pubmed: 16856488] | To establish a comprehensive HPLC analytical method of Huanglianjiedu decoction.
METHODS AND RESULTS:
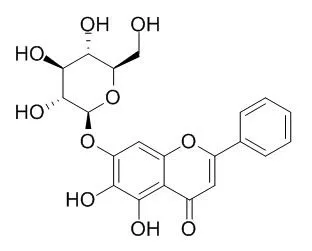
This study was performed by HPLC-UV/MS to identify the chemical constituents of the whole and individual herbs of the "Huanglianjiedu decoction". Zorbax Extend C18 (150 mm x 4. 6 mm ID, 5 microm) column was used; the mobile phase was composed of acetonitrile (A) and water (B, with 0.5% acetic acid) with gradient elution; the flow rate was 1.0 mL x min(-1) and the column temperature was setup at 25 degrees C. The detection wavelength was 254 nm. The chromatogram of Huanglianjiedu decoction showed 21 main peaks. Peaks 1, 2, 5 and 18 were from Gardenia jasminoides Ellis, Peaks 8, 13, 14, 15, 16, 17, 19 and 21 from Scutellaria baicalensis Georgi. While 10 from Coptis chinensis Franch and 20 from Phellodendron amurense Rupr., Peaks 3, 4, 6, 9, 11 and 12 came from them together. Peak 7 presented in the chromatograms of the herbs except Gardenia jasminoides Ellis. By comparison of the retention time, the on-line UV spectra and MS spectra, 11 peaks were identified as 5 (geniposide), 9 (jatrorrhizine), 10 (coptisine), 11 (palmatine), 12 (berberine), 13 (baicalin), 15 (Oroxin A), 17 (wogonoside), 19 (baicalein), 20 (obaculactone), 21 (wogonin), then eight of them were quantified by HPLC-UV.
CONCLUSIONS:
The method could represent the characteristics of Huanglianjiedu decoction, and it could be used to evaluate the quality and quantity of Huanglianjiedu decoction. It distinguished between Coptis chinensis Franch and Phellodendron amurense Rupr. by HPLC for the first time. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)