| In vitro: |
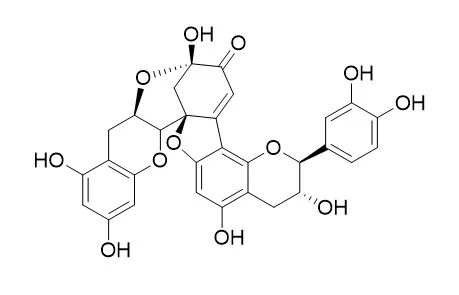
| J Agric Food Chem. 2002 Feb 27;50(5):1218-1224. | | Comparative study of the products of the peroxidase-catalyzed and the polyphenoloxidase-catalyzed (+)-catechin oxidation. Their possible implications in strawberry (Fragaria x ananassa) browning reactions[Pubmed: 11853507] | | The peroxidase- and polyphenoloxidase-catalyzed oxidations of (+)-catechin yield several products showing different degrees of polymerization, which are apparently responsible for the pigment decay and the associated browning reaction that occurs in processed strawberry fruits and their derived foods. In this work, we have purified both peroxidase and polyphenoloxidase from Oso Grande cv. strawberry fruits, and comparatively analyzed the products of their enzyme-mediated (+)-catechin oxidation. The joint analysis by reversed-phase and size-exclusion HPLC of the (+)-catechin oxidation products obtained with both enzymes indicate that they were qualitatively the same: dehydrodicatechin B4, a (+)-catechin quinone methide, Dehydrodicatechin A, a (+)-catechin trimer, and a (+)-catechin oligomer with polymerization degree equal to or greater than 5. The main quantitative differences between the oxidative reactions were the great amount of oligomer formed in the case of the polyphenoloxidase-mediated reaction and the low amount of (+)-catechin reacted in the case of the peroxidase-mediated reaction. One of the possible reasons for such low levels of (+)-catechin consumption in the case of the peroxidase-mediated reaction was the possible inhibition by products of the enzyme-catalyzed oxidation. In fact, the peroxidase-mediated (+)-catechin oxidation was differentially inhibited by Dehydrodicatechin A, showing a competitive type inhibition and a k(I) of 6.4 microM. In light of these observations, these results suggest that brown polymer formation, estimated as oligomeric compounds resulting from (+)-catechin oxidation, in strawberries is mainly due to polyphenoloxidase, and although peroxidase also plays an important role, it is apparently auto-regulated by product (Dehydrodicatechin A) inhibition. | | Z Naturforsch C J Biosci . Sep-Oct 2007;62(9-10):656-660. | | Evaluation of cytotoxic compounds from calligonum comosum L. growing in Egypt[Pubmed: 18069236] | | Calligonum comosum (Polygonaceae), an Egyptian desert plant, was extracted and fractionated using petroleum ether, methylene chloride, and ethyl acetate. The total methanolic extract and other fractions were tested for their anticancer activity using Ehrlich ascites, brine shrimp and antioxidant assays. Ethyl acetate fraction proved to be the most active in all assays. Eight compounds were isolated, purified, and identified from this fraction as (+)-catechin (1), Dehydrodicatechin A (2), kaempferol-3-O-rhamnopyranoside (3), quercitrin (quercetin-3-O-rhamnopyranoside) (4), beta-sitosterol-3-O-glucoside (5), isoquercitrin (quercetin-3-O-glucopyranoside) (6), kaempferol-3-O-glucuronide (7), and mequilianin (quercetin-3-O-glucuronide) (8). All isolated compounds were tested for their cytotoxicity and antioxidant activity. Compound 2 showed the best cytotoxic and antioxidant activity. | | J Sep Sci . 2021 Dec;44(24):4422-4430. | | Isolation and purification of flavonoids from Euonymus alatus by high-speed countercurrent chromatography and neuroprotective effect of rhamnazin-3-O-rutinoside in vitro[Pubmed: 34670011] | | The flavonoids from Euonymus alatus exhibit many biological activities including significant antioxidant, anti-inflammatory, anti-cancer. In this work, a high-speed countercurrent chromatography method for the isolation and purification of flavonoids from crude extracts of Euonymus alatus was established. The effects of several solvent systems on the separation efficiency of target compounds in the extract of Euonymus alatus were studied. The solvent system composed of n-hexane-ethyl acetate-methanol-water at a volume ratio of (3:5:3:5, v/v) was chosen, in which the lower phase was used as the mobile phase at the rotation speed of 800 rpm and flow rate of 2.0 mL/min. The three flavonoids were obtained and identified as patuletin-3-O-rutinoside, rhamnazin-3-O-rutinoside, and Dehydrodicatechin A by mass spectroscopy and nuclear magnetic resonance, and the quantities of patuletin-3-O-rutinoside, rhamnazin-3-O-rutinoside, and Dehydrodicatechin A were 2.2, 9.7, and 1.8 mg, respectively. The results indicated that high-speed countercurrent chromatography was a simple and efficient method for the isolation and purification of flavonoids from the crude extracts of Euonymus alatus. The cellular antioxidant activity experimental result indicated that rhamnazin-3-O-rutinoside could alleviate H2 O2 -induced oxidative stress. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)